RESEARCH ARTICLE
Antifungal Activities and Characterization of Some New Environmentally Safe Cu (II) Surfactants Substituted 2-Amino-6-Methyl Benzothiazole
Arun Kumar Sharma1, *, Rashmi Sharma2, Antima Gangwal2
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 1
Last Page: 11
Publisher Id: PHARMSCI-5-1
DOI: 10.2174/1874844901805010001
Article History:
Received Date: 22/03/2018Revision Received Date: 10/05/2018
Acceptance Date: 11/05/2018
Electronic publication date: 16/07/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Biologically potent compounds are one of the most important classes of materials for the upcoming generations.
Objectives:
Increasing number of microbial infectious diseases and resistant pathogens create a demand and urgency to develop novel, potent, safe and improved variety of antimicrobial agents.
Methods:
The copper surfactants substituted 2-amino-6-methyl benzothiazole were synthesized. The synthesized complexes have been characterized by IR, NMR, ESR spectroscopic methods. The antifungal activities have been evaluated by testing against Alternaria alternate fungi. All complexes showed good antifungal activity because chelation increases the anti-microbial potency.
Result:
The studies suggest that the copper (II) ions in soaps may be responsible for the enhancement of the activity against fungi. The evaluation of anti -fungal studies further revealed that fungitoxicity of the complexes also depends on the nature of metal ions. The chelation reduces the polarity of central metal ion mainly because of partial attaining of its positive charge with the donor groups and possible π- electron delocalization over the whole chelate ring. Such chelation increases the lipophilic character of the central atom, which subsequently favors its permeation through the lipoid layer of the cell membrane. Their efficiency increases with their concentration.
1. INTRODUCTION
Colloidal surfactants have become one of the most important requirements in day to day life, and have great importance in modern engineering and pharmacological fields. Many copper thiosemicarbazone complexes have been found to have significant anti-tubercular, fungicidal and antitumor activities [1, 2]. The use of copper linoleate as heavy duty wood preservative and many other biological activities of copper metal-containing surfactants have also been studied [3, 4]. Recently, the deeper understanding of the role of metal ion in bio-system has led to the awareness that metal complexing is useful in the treatment of bacterial, fungicidal and viral diseases [5, 6].Many copper compounds are employed as pesticides either alone (as Cu (I) oxide and Cu (II) sulfate) or in mixtures [7]. Formulated proprietary brands of cuprous oxide are extensively employed as fungicides and seed dressings. Copper oxychloride has a number of applications, by far the most important being as an agricultural fungicide for which purpose it is extensively employed in formulated form as dust, wettable powders and pastes [8-10]. The effect of silver nanoparticles on various fungi has been reported recently [11, 12]. Therefore, a substantial research is needed for their discovery and improvement. Chemistry of present era aims to build a pollution free environment. For the same, it targets to create some alternatives which are eco-friendly and nature loving. The present research work is a step towards achieving such alternatives. For this, the copper surfactants substituted 2-amino-6-chloro benzothiazole were synthesized. The antifungal activities of these have been evaluated by testing against Alternaria alternate. The fungitoxicity results indicate that the strain of fungal species are susceptible towards these and suggests that with the increase in the concentration of copper surfactant and its complexes, it may increase further.
2. EXPERIMENTAL
The acids (n–caprylic acid, n–capric acid and n–lauric acid) have been purified by keeping over anhydrous sodium sulphate for a week and then distilled under reduced pressure. The purity of acids was checked by the determination of their melting points. n - Caprylic acid (16.3 oC) n - Capric acid (31.2 oC), n - Lauric acid (44.0 oC). Copper soaps were prepared by direct metathesis of the corresponding sodium soap with a slight excess of the required amount of copper sulphate solution at 50-55oC. After washing with hot water and alcohol, the samples were dried at 100-105oC. Finally, under reduced pressure, they were recrystallized twice from hot benzene. Ligands were synthesized using thiocyanation method [13]. The purity of the benzothiazoles has been checked by thin layer chromatography in various non-aqueous solvent systems. The metal was analysed by standard procedure. C, H, N, Cl and S analyses were performed at RSIC, CDRI Lucknow. In general, all the solid complexes (about 90% yield) with bluish green periphery were obtained (Table 1). These are soluble in ethanol, methanol, benzene and other organic solvents and insoluble in water. All the complexes are quite stable at room temperature up to 170oC. On the basis of elemental analysis, the complexes have been assigned the composition Cu2(CnH2n+1COO-)4L2, n=7,9,11 and suggested 1:1 type stoichiometry (Figs. (1-3) [14].
 |
Fig. (1). Synthesis of ligand by thiocyanation method. |
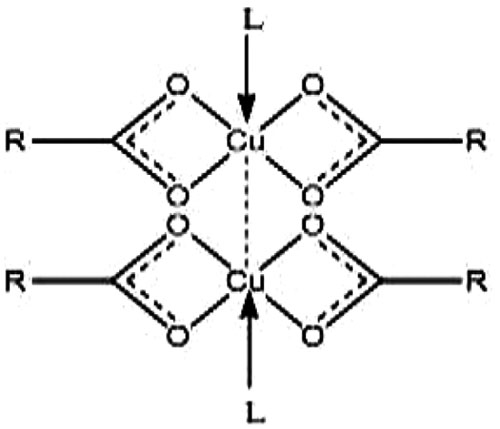 |
Fig. (2). Proposed structure of copper soap. |
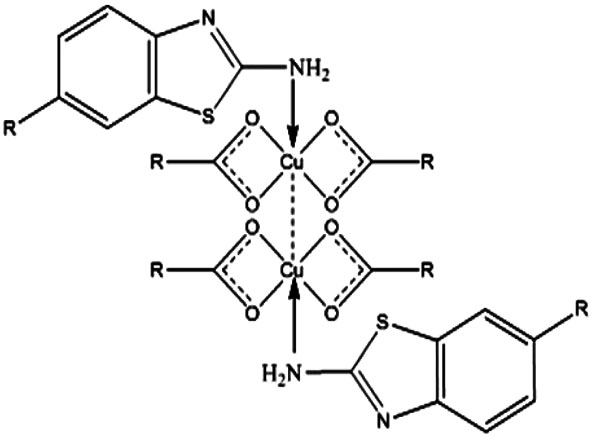 |
Fig. (3). Proposed structure of copper soap complex. |
2.1. Fungicidal Activities:
1 ml of the soap solution was aseptically transferred into sterile petri plates. Into these plates, 20 ml of P.D.A. was poured and was mixed with soap solution by rotating the petri plates in clockwise and anticlockwise direction 3-4 times and was allowed to solidify. After the solidification of the above medium, single hypha / spore of test organism was aseptically transferred in the centre of the petri plates. The plates were incubated at 30 oC for 3 days. After the period of incubation, the plates were observed for the growth of fungus in different concentration of the soap solution used in the present study. The data were statistically analyzed according to the following formula [15].
% Inhibition = (C-T)*100/C
Where C = diameter of fungal colony in control plates after 3 days
T = diameter of fungal colony in test plate after 3 days
3. CHARACTERIZATION
In order to study the structure of complexes, the infrared absorption spectra of compounds were obtained on a Perkin–Elmer 5100.4367 Spectrophotometer [4000-200 cm-1] and ESR analysis was made on Marker-TCNE analyser at RSIC, IIT Powai, Mumbai. 1H NMR spectra (C6D6) was recorded on an NMR spectrometer at RSIC, IIT Powai, Mumbai. The ESR spectra of the complex recorded on X-band at frequency 9.07 GHz under the magnetic field strength of 2000 ± 4000 and 3000 ± 2000 gauss at room temperature. ESR Spectrum is characterized by the position intensity and shape as to component lines. The position of ESR is referred as g values and is directly determined by the energy levels. The variation in g value is interpreted in terms of the first and second order spin-orbit interaction. The g values for ESR signal was calculated by the formulae [16].
g = hν/βH
Where,
ν = Freq. of band in kHz. β= Bohr magneton
H = magnetic field
h = Planck’s constant
4. RESULTS AND DISCUSSION
4.1. IR Studies
The detailed infrared spectral investigation reveals that there are marked differences between the spectra of pure copper soaps and pure ligand than those of the corresponding complexes (Table 2) The absorption bands observed in the region 2960-2950 cm-1 and 2900-2950 cm-1 respectively were assigned to the antisymmetric stretching for methyl group of the soap segment present in the complex. Symmetric stretching bands for methyl group has been assigned in the region 2820-2850 cm-1. It has been observed that the absorption peaks, which are characteristics of the aliphatic portion of the soap molecules, remain unchanged on going while being transferred from soaps to the complexes. The absorption spectra in the region 710-725 cm-1 may probably be due to the rocking vibration of a chain of methylene group –(CH2)n. The peaks in the region 1575-1590 cm-1 and 1400-1410 cm-1 are due to COO-, C-O anti-symmetric and symmetric stretching, respectively [17].
The presence of bands in the region 810±20 cm-1 was due to C-H deformation (in plane) present in the tri-substituted benzene ring of benzothiazole segment, where two hydrogens are adjacent [1,2,and 4 position]. In 2-amino-6-methyl benzothiazole, the C-H deformation vibration of –CH3 group has been observed at 1477 cm-1. C-N stretching (aryl tertiary) peaks was observed in the region 1360-1310 cm-1. C=N stretching peaks in the region 1460-1430 cm-1 was also observed. The C-S stretching bands present in the region of 700-600 cm-1 also confirm the structure of the molecule. IR spectrum of the pure ligand 2-amino-6-R benzothiazole [R= -CH3] contained peaks in the region 3450±15 cm-1 due to symmetric N-H stretching, which shifted to 3400±20 cm-1 on complexation, indicating the participation of the primary amino group in the chelation (Table 3) [18].
4.2. NMR Studies
The 1H NMR spectra of the complexes were collected in C6D6 and examined to determine bonding and tautomerism present in the structure. A perusal of the spectra of the complexes shows aliphatic –CH3 proton signal at δ - 0.9 and -CH2 proton signal at δ - 1.2 of soap segment, indicating the presence of soap segment in the complex molecule.
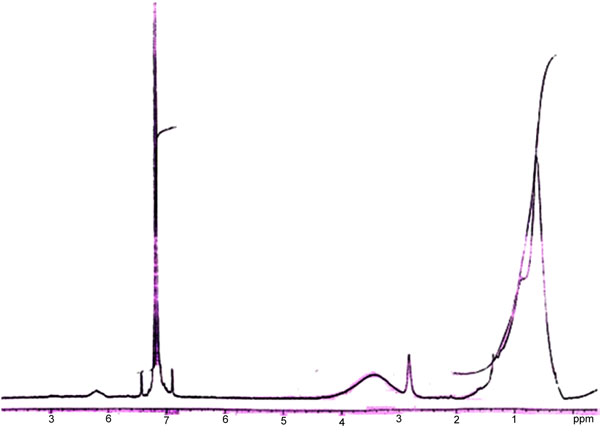 |
Fig. (4). NMR spectra of ligand (2-amino-6-methyl benzothiazole). |
A broadened peak has been observed in all the spectra of the complexes at δ 3.2 – 4.2 due to the presence of aromatic –NH2 protons. In the given spectra of CCprAMB (Fig. 4), the above peak was clear which indicates the coordination through the –NH2 group of the benzothiazole segment to the metal atom of soap segment. The proton present in the nitrogen atom may undergo rapid, intermediate or slow exchange. The broadening of the observed peak is suggestive of a slow exchange because the electrical quadripole moment of the nitrogen nucleus induces a moderately efficient spin relaxation. A very weak signal was observed at δ - 8.5 in the spectra, which may be due to tautomerism present in the molecule of the complex similar to the pyrrole and indole. The triplets at δ 6.9 – 7.2 are may be due to tri-substituted aromatic protons of benzothiazole segment indicating that the environment of all the three protons is different. Another peak present at δ - 2.8 in the methyl substituted complex confirmed the presence of aromatic methyl group in the benzothiazole segment of the molecule suggesting a downfield shift [19].
4.3. ESR Studies
Literature reveals that magnetic conductivity and spectral studies of complexes of some first-row transition elements with pyrazine carboxylic acid have been conducted , where it acts as a bidentate ligand through O and N donor atoms [20].
| Molecular Formula of Complex | Colour | Yield | M.P. | Cu | C | H | O | N | S |
|---|---|---|---|---|---|---|---|---|---|
| Cu2(C7H15COO-)4. (C8H8N2S)2 CCplAMB |
Dark Greenish Blue | 92% | 210 | 12.28 [12.36] |
55.92 [56.09] |
7.26 [7.40] |
12.39 [12.45] |
5.37 [5.45] |
6.19 [6.23] |
| Cu2(C9H19COO-)4. (C8H8N2S)2 CCprAMB |
Greenish Blue | 89% | 220 | 10.80 [11.15] |
58.87 [58.99] |
8.01 [8.08] |
11.15 [11.24] |
4.80 [4.92] |
5.52 [5.62] |
| Cu2(C11H23COO-)4. (C8H8N2S)2 CLrtAMB |
Blue | 91% | 225 | 9.95 [10.15] |
61.18 [61.39] |
8.59 [8.63] |
10.15 [10.23] |
4.34 [4.47] |
5.03 [5.11] |
| Assignment | Soap | Ligand | ||
|---|---|---|---|---|
| Cu-Caprylate | Cu-Caprate | Cu-Laurate | 2-amino-6-methyl benzothiazole |
|
| N-H stretching in amino group | --- | --- | --- | 3434.9 |
| CH3, C-H antisym. Stretching | 2900.0 | 2950.0 | 2965.0 | --- |
| CH2, C-H symmetric stretching | 2820.0 | 2840.0 | 2855.0 | --- |
| COO-, C-O antisym. Stretching | 1575.0 | 1590.0 | 1605.0 | --- |
| C-H deformation vibration of –CH3 group | --- | --- | --- | 1477.9 |
| C=N stretching | --- | --- | --- | 1460.0 |
| CH2, deformation | 1430.0 | 1430.0 | 1430.0 | --- |
| COO-, C-O symmetric | 1410.0 | 1410.0 | 1410.0 | --- |
| C-N stretching (aryl tertiary) | --- | --- | --- | 1350.0 |
| CH2 twisting and wagging | 1295.0 | 1275.0 1230.0 |
1260.0 | --- |
| CH3 rocking | 1105.0 | 1110.0 | 1115.0 | --- |
| C-H deformation (in plane) due to benzene ring | --- | --- | --- | 808.1 |
| CH2 rocking | 710.0 | 718.0 | 726.0 | --- |
| C-S stretching | --- | --- | --- | 680.0 |
| Assignment | CCplAMB | CCprAMB | CLrtAMB |
|---|---|---|---|
| N-H stretching in amino group | 3440.1 | 3419.2 | 3419.2 |
| CH3, C-H antisym. Stretching | 2932.6 | 2958.7 | 2922.1 |
| CH2, C-H symmetric stretching | 2864.5 | 2864.5 | 2848.8 |
| COO-, C-O antisym. Stretching | 1590.0 | 1560.0 | 1575.0 |
| C-H deformation vibration of –CH3 group | 1515.0 | 1520.0 | 1470.0 |
| C=N stretching | 1425.6 | 1420.3 | 1415.1 |
| C-N stretching (aryl tertiary) | 1341.9 | 1336.6 | 1350.0 |
| CH3 rocking | 1116.9 | 1111.6 | 1100.0 |
| C-H deformation (in plane) due to benzene ring | 829.1 | 815.0 | 805.0 |
| CH2 rocking | 705.0 | 740.0 | 724.4 |
| C-S stretching | 682.6 | 672.1 | 635.5 |
The ESR spectra of the complexes synthesized by us were recorded on X-band at frequency 9.07 GHz under the magnetic field strength of 2000±4000 and 3000±2000 gauss at room temperature. The value of ESR parameters for all the complexes is given in Table 4. A perusal of Table 4 shows that the values of g, gII, 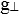 are greater than the value of g0 ii.e. 2.0027. This indicates that the distortion from the regular octahedron has taken place in the shape of the complex. Also the trend gII>
are greater than the value of g0 ii.e. 2.0027. This indicates that the distortion from the regular octahedron has taken place in the shape of the complex. Also the trend gII> for all the complexes indicates that the unpaired electron is most likely in dx2-y2 orbital of Cu (II) giving 2B1g as the ground state. This fact supports that complexes possess elongated octahedral geometry. It is well known that gII is a moderately sensitive function for indicating covalency. Thus, the values of gII are suggestive of the covalent character of metal-ligand bond [21, 22]. Earlier magnetic studies, about copper (II) soaps confirm the binuclear configuration in the solid state. However, the low values of the magnetic moments for copper ion present in copper (II) soaps in non-aqueous solvent like alcohol and hydrocarbon confirm that the binuclear configuration exists in the soap solutions and the dispersion unit is a binuclear molecule having the structure proposed in Figs. (5-7) [23, 24].
for all the complexes indicates that the unpaired electron is most likely in dx2-y2 orbital of Cu (II) giving 2B1g as the ground state. This fact supports that complexes possess elongated octahedral geometry. It is well known that gII is a moderately sensitive function for indicating covalency. Thus, the values of gII are suggestive of the covalent character of metal-ligand bond [21, 22]. Earlier magnetic studies, about copper (II) soaps confirm the binuclear configuration in the solid state. However, the low values of the magnetic moments for copper ion present in copper (II) soaps in non-aqueous solvent like alcohol and hydrocarbon confirm that the binuclear configuration exists in the soap solutions and the dispersion unit is a binuclear molecule having the structure proposed in Figs. (5-7) [23, 24].
| Name of Complex and Molecular Formula | g | gII |  |
|---|---|---|---|
| CCplAMB Cu2(C7H15COO-)4*(C8H8N2S)2 |
2.0184 | 2.2282 | 2.1282 |
| CCprAMB Cu2(C9H19COO-)4*(C8H8N2S)2 |
2.0195 | 2.2349 | 2.1090 |
| CLrtAMB Cu2(C11H23COO-)4*(C8H8N2S)2 |
2.0270 | 2.2202 | 2.1092 |
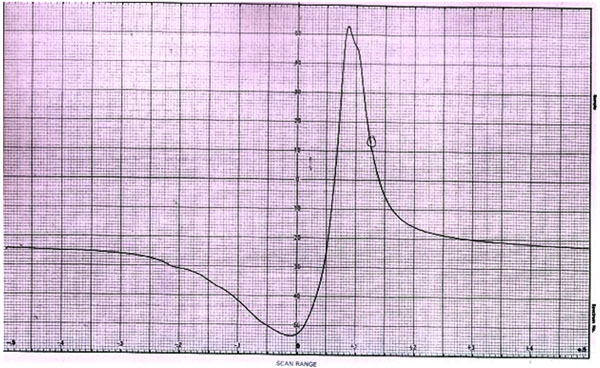 |
Fig. (5). ESR spectra of complex CCplAMB. |
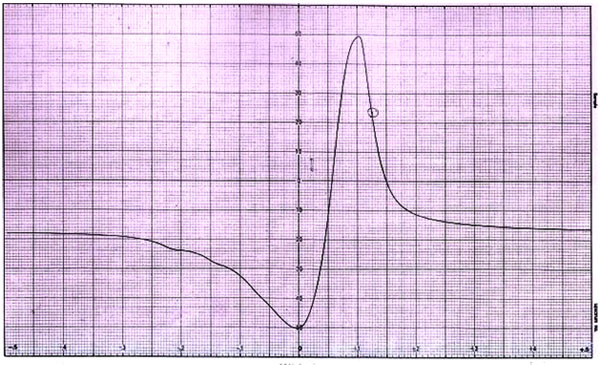 |
Fig. (6). ESR spectra of complex CCprAMB. |
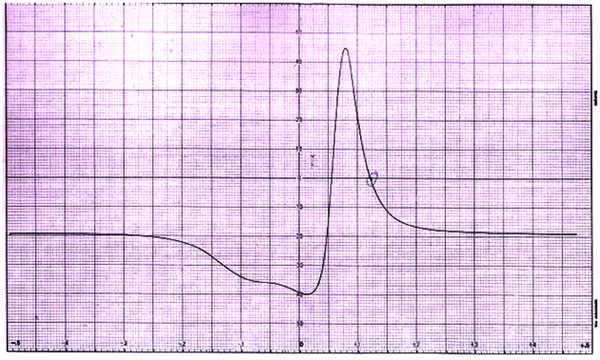 |
Fig. (7). ESR spectra of complex CLrtlAMB. |
4.4. Fungicidal Activities
The antifungal activities of the pure ligands, pure soaps and their corresponding complexes have been evaluated by testing against Alternaria-alternata [25-26] at a different concentration by Agar plate technique. A perusal of Fig. (8) reveals that all complexes show higher activity than pure soaps and ligands suggesting that complexes are more powerful antifungal agents and benzothiazole and other N, S, O etc [27, 28]. containing compounds are able to enhance the performance of copper soaps. The organic compounds containing amino group play an important role in biology, as it constitutes the repeating unit of the polypeptide macromolecules. These results show that the Cu (II) soap-complexes of ligands are much more toxic than the ligands themselves [29]. The enhanced activity of newly synthesized complexes as compared to those of the ligand can possibly be explained on the basis of chelate formation, the presence of donor atoms, basicity as well as the structural compatibility with molecular nature of the toxic moiety [30]. The enhanced biological activity of complexes is in accordance with the chelation theory. The results of ANOVA [31] for the antifungal activities for all soaps complexes are shown in Table 5. The predicted R2 was obserbed to be in reasonable agreement and closer to1.0. This confirms that the experimental data are satisfactory. The descriptive statics results shown in Table 6 confirm the satisfactory results in triplet [32].
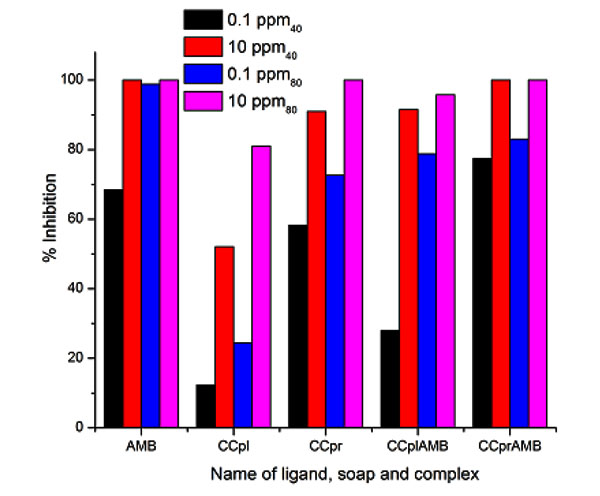 |
Fig. (8). Antifungal activity of ligand soap and complex (methyl) for fungi Alternaria Alternata. |
| Compound | conc. (ppm) | Count | Average % Inhibition | Variance | Coefficient Variance | Std. Deviation | Std. Error |
|---|---|---|---|---|---|---|---|
| CCplS40 | 0.1 | 3 | 12.4 | 0.015 | 0.12 | 0.072 | 1.007 |
| 10 | 3 | 52.22 | 0.012 | 0.11 | 0.063 | 0.21 | |
| CCplS80 | 0.1 | 3 | 24.36 | 0.043 | 0.2 | 0.12 | 0.854 |
| 10 | 3 | 80.5 | 0.16 | 0.04 | 0.23 | 0.496 | |
| CCprS40 | 0.1 | 3 | 58.2 | 0.01 | 0.17 | 0.1 | 0.057 |
| 10 | 3 | 90.76 | 0.023 | 0.16 | 0.152 | 0.088 | |
| CCprS80 | 0.1 | 3 | 72.55 | 0.013 | 0.15 | 0.115 | 0.066 |
| 10 | 3 | 99.99 | 0.015 | 0.01 | 0.01 | 0.005 | |
| AMB40 | 0.1 | 3 | 68.23 | 0.013 | 0.16 | 0.115 | 0.066 |
| 10 | 3 | 99.99 | 0.01 | 0.1 | 0.101 | 0.058 | |
| AMB80 | 0.1 | 3 | 98.8 | 0.002 | 0.05 | 0.051 | 0.028 |
| 10 | 3 | 99.99 | 1.00E-04 | 0.01 | 0.011 | 0.005 | |
| CCplAMB40 | 0.1 | 3 | 27.99 | 0.01 | 0.36 | 0.102 | 0.058 |
| 10 | 3 | 91.43 | 0.033 | 0.19 | 0.182 | 0.105 | |
| CCplAMB80 | 0.1 | 3 | 78.74 | 0.029 | 0.21 | 0.172 | 0.099 |
| 10 | 3 | 95.67 | 0.012 | 0.11 | 0.111 | 0.064 | |
| CCplAMB40 | 0.1 | 3 | 77.4 | 0.01 | 0.13 | 0.1 | 0.058 |
| 10 | 3 | 99.94 | 0.01 | 0.1 | 0.103 | 0.06 | |
| CCplAMB80 | 0.1 | 3 | 83.27 | 0.002 | 0.05 | 0.046 | 0.026 |
| 10 | 3 | 99.99 | 3.33E-05 | 0.005 | 0.005 | 0.003 |
| Name of compound | SS | df | MS | F | P-value | F crit. | R- SQUARE |
|---|---|---|---|---|---|---|---|
| CCplS | 8408 | 3 | 2802 | 445508 | 3.16E-21 | 4.06 | 0.992 |
| CCprS | 3144 | 3 | 1048 | 96521 | 1.43E-18 | 4.06 | 0.995 |
| AMB | 2212 | 3 | 737 | 112762 | 7.7E-19 | 4.06 | 0.999 |
| CCplAMB | 8734 | 3 | 2911 | 135740 | 3.67E-19 | 4.06 | 0.994 |
| CCprAMB | 1207 | 3 | 402 | 69685 | 5.28E-18 | 4.06 | 0.998 |
CONCLUSION
In conclusion, it can be said that the appearance of enhanced activity may be due to synergistic mechanism i.e. the free ligands are less active but on complexation, show more activity in combination with copper (II) soaps. Thus it is evident that concentration plays a vital role in increasing the degree of inhibition. So fungicidal screening data revealed that at lower concentration, the inhibition of growth is less as compared to higher concentration.In all complexes, toxicity increases with the domination of methanol in the solvent mixture of methanol and benzene [40% methanol–benzene and 80% methanol–benzene]. Suggesting that complexes are more effectively playing their role in determining the growth of fungi in the domination of polar solvent in the ternary system: complex + methanol + benzene. Some recent studies about antimicrobial activities of newly synthesized 1, 4-benzothiazines possessing thiazolyl / imidazolyl moieties also support our findings.
Acknowledgment-The authors pay their sincere gratitude to, Principal, S. P. C. Govt. College, Ajmer, S.D. Govt. College Beawar, Rajasthan (India) for providing necessary research facilities to accomplish this study.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Decleared none.














