RESEARCH ARTICLE
Synthesis, Characterization and Antimicrobial Evaluation of Novel Mannich Bases Containing Pyrazole-5-One Phosphonates
V. E. Rani*, L. K. Ravindranath
Article Information
Identifiers and Pagination:
Year: 2016Volume: 3
First Page: 49
Last Page: 55
Publisher Id: PHARMSCI-3-49
DOI: 10.2174/1874844901603010049
Article History:
Received Date: 3/4/2015Revision Received Date: 5/4/2016
Acceptance Date: 05/04/2016
Electronic publication date: 18/05/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Background:
Newly synthesised compounds of phosphonates were prepared by condensation of diethylphosphate with imine which undergoes a reaction of mannich bases with pyrazole containing schiffs base. The base was prepared by condensation of aldehyde with primary amine. These newly synthesised derivatives were characterised by spectral analysis.
Objective:
Mannich bases are very important to synthesize wide variety of natural products and pharmaceuticals.
Method:
Thin Layer Chromatography was performed on aluminum sheet of silica gel 60F254, E-Merk, Germany using iodine as visualizing agent. IR Spectra were recorded as KBr pellets on Perkin-Elmer 1000 units, instruments. All 1H and 13C-NMR spectra were recorded on a Varian XL-300 spectrometer operating at 400MHz and 75 MHz. 31P-NMR spectra were recorded on a Varian XL-spectrometer operating at 161.89MHz. The compounds were dissolved in dimethylsulfoxide and Chemical shifts were referenced to Trimethylsilane (1H and 13C-NMR) and 85% phosphoric acid (31P-NMR).
Results:
Some of the novel synthetic compounds of Pyrazole Mannich base-Phosphonates showed great potential in field of medicinal chemistry and good biological activity.
Conclusion:
It can be concluded that this class of compounds certainly holds great potential for the discovery of novel classes of antimicrobial agents.
INTRODUCTION
Pyrazole-5-one derivatives possess various types of biological activities. It is due to their wide use in medicinal chemistry some of them possess anti-tuberculosis, anti-neoplastic, anti-diabetic, anti-fertility, anti-thyroid and anti microbial activity [1-4].
The chemistry of phosphorus heterocyclic compounds containing nitrogen has pioneered the application of combinatorial techniques to the development of new pharmaceutical materials with novel properties. Organophosphorus compounds possess significant biological activity against broad spectrum of bacteria, pets, virus, fungicides and plant growth regulators. Some Organophosphorus compounds have been described in the literature as inhibitors of bacterial [5], herbicides, insecticides, pesticides [6, 7], anti-fungal agents [8], anti-HIV [9], anti-cancer[10], anti-viral and anti-inflammatory[11].
A good deal of importance was given to Phosphonate derivatives in the field of organophosphorus heterocyclic chemistry due to their unique biological applications [12]. In view of the above observations, we synthesized Mannich bases containing Pyrazole-5-one Phosphonates and screening for possible biological and pharmacological activities.
RESULTS AND DISCUSSION
Chemistry
All the chemicals used in the present investigation were purchased from Sigma-Aldrich Chemicals company, Inc. USA. And used without further purification. TLC was performed on aluminium sheet of silica gel 60F254, E-Merk, Germany using iodine as visualizing agent. Melting points were determined in open capillary tubes on Mel-Temp apparatus and are uncorrected. Column chromatography was performed on silica gel with different solvent systems as eluents to afford the pure compound. The IR Spectra were recorded as KBr pellets on Perkin-Elmer 1000 units, instruments. All 1H and 13C-NMR spectra were recorded on a Varian XL-300 spectrometer operating at 400MHz for 1H -NMR and 75 MHz for 13C-NMR. 31P-NMR spectra were recorded on a Varian XL-spectrometer operating at 161.89MHz. The compounds were dissolved in DMSO-d6 and Chemical shifts were referenced to TMS (1H and 13C-NMR) and 85% H3PO4 (31P-NMR). Mass spectral data was recorded on FAB-MS instrument at 70ev with direct inlet system. Elemental analysis was recorded on a Carlo Erba 1108 elemental Analyzer, Central Drug Research Institute, Lucknow, India.
CONCLUSION
The newly synthesized compounds Diethyl ((4-fluorophenyl)/(4-chlorophenyl)/(4-bromophenyl)/(4-(trifluoro-methyl) phenyl) amino) (3-methyl-1-(morpholino methyl)/(4-methylpiperazin-1-yl) -5- oxo-4, 5-dihydro-1H-pyrazol-4-yl) methyl) phosphonates 6(a-h) were found to be active in the study of antifungal activity. It can be concluded that this class of compounds certainly holds great promise towards the pursuit to discover novel classes of antimicrobial agents.
EXPERIMENTAL SECTION
General Procedure A for Preparation of 3(a-d)
The quantity of 4-fluoro aniline (2.2gr, 0.020 mole) (2a) and 3 - Methyl - 5 - oxo - 4, 5 - dihydro - 1H - pyrazole - 4 - carbaldehyde (1.746gr, 0.014mole) (1) were dissolved in absolute alcohol, to this three drops of acetic acid is added then heated on a steam bath for 5-6 hours at 100°C. After standing for 24 hours at room temperature, the crude product was purified by column chromatography (60-120 mesh silica gel,eluent: 10% EtoAc pet ether). Finally, the product compound 4 - (((4 - fluorophenyl) imino) methyl) - 3 - methyl - 1H - pyrazol - 5(4H) - one (3a) which was recrystallized from warm absolute alcohol. Yield 75%, m p 153-155°C.
The similar procedure was adopted to synthesize 3(b-d) (2.7gr, 0.011mol - 3b, 3.1gr, 0.011mol-3c, 2.9gr, 0.018mol-3d) by condensing 3-Methyl-5-oxo-4, 5-dihydro-1H-pyrazole-4-carbaldehyde (1) with 4-chloro aniline (2.5gr, 0.020 mol-2b), 4-bromo aniline (3.44gr, 0.20mol-2c) and 4-trifluoro aniline (3.22gr, 0.020mol-2d) respectively.
General Procedure B for Preparation of 5(a-h)
A mixture of 4-(((4-fluorophenyl) imino) methyl)-3-methyl-1H-pyrazol-5(4H)-one(0.87gr, 0.004mol) (3a), morpholine (0.78gr, 0.09mol) (4a) (0.15 mol) and water 20 ml was stirred to obtained a clear solution. To this solution, HCHO (0.05 mol) and DMF were added in ice cold condition and stirred for 2 hours in an ice bath and left over night at room temperature. The progress of the reaction was monitored by TLC using cyclohexane and ethylacetate (7:3) solvent mixture as a mobile phase. At the end of the reaction, the mixture was taken in a 30 ml dichloromethane and neutralized with 50 ml 1N NaOH solution, after neutralization the mixture was extracted with CH2Cl2 (3(25 ml). The combined extract was dried on Na2SO4. After filtration, the solvent was removed with rota evaporator. The residue was purified by column chromatography, using 60-120 mesh silica and CHCl3 solvent was used as an elutent. Finally the product compound 4 - (((4 - fluorophenyl) imino) methyl) - 3 - methyl - 1 - (morpholinomethyl) - 1H - pyrazol - 5(4H) - one(0.891gr, 0.0028mol) (5a) was purified from aqueous dimethyl formamide. Yield 70%, m p 162-164°C.
The similar procedure was adopted to synthesize 5(b-h) (1.305gr, 0.003mol-5b, 1.32gr, 0.0035mol-5c, 1.28gr,0.0035mol-5d, 1,15gr, 0.0035mol-5e, 1.13gr, 0.0032mol-5f, 1.27gr, 0.0032mol-5g, 1.23gr, 0.0032mol-5h) by condensing 3(a-d) (0.87gr, 0.004mol-3a, 1.414gr, 0.006mol-3b, 1.40gr, 0.005mol-3c, 1.34gr, 0.005mol-3d) with morpholine (0.78gr, 0.009mol) (4a) N-methylpiperazine (0.90gr, 0.005mol) (4b) respectively.
General Procedure C for Preparation of 6(a-h) (Scheme-1)
A mixture of 4-(((4-fluorophenyl) imino) methyl)-3-methyl-1-(morpholinomethyl)-1H- pyrazol-5(4H)-one(0.95gr, 0.003mol) (5a) and Diethyl phosphite (1.24 ml, 0.009 mol) in anhydrous toluene (15ml) was added dropwise. Stirring was continued at room temperature for another 0.5 hour, after which the mixture was heated under reflux for 4-6 hours. The reaction was monitored by TLC on silica gel using petroleum ether-ethyl acetate (1:2 v/v). After completion of the reaction, the solvent was removed by rota evaporator and the resulting residue was purified by column chromatography on silicagel (100-200 mesh) and ethyl acetae-hexane, (3:7 ratio) as an eluent to afford pure Diethyl (((4-fluorophenyl) amino) (3-methyl -1- (morpholinomethyl) -5-oxo -4,5-dihydro-1H-pyrazol-4-yl) methyl) phosphonates(0.95gr, 0.002mol) (6a), was purified from aqueous dimethyl formamide. Yield 70%, m p 176-178°C.
The similar procedure was adopted to synthesize 6(b-h) (0.96gr, 0.002mol-6b, 1.03gr, 0.002mol-6c, 0.98gr, 0.001mol-6d, 0.98gr, 0.002mol-6e, 1.00gr, 0.002mol-6f, 1.00gr, 0.001mol-6g, 1.16gr, 0.002mol-6h) by the reaction between 5(b-h) with Diethyl phosphite. The structure of these newly synthesized compounds of 6(a-h) were established by IR, 1H-NMR, 13C-NMR, 31P-NMR, and mass data and elemental analysis.
Diethyl (((4-fluorophenyl) amino) (3 - methyl - 1 - (morpholinomethyl) - 5 - oxo - 4, 5 - dihydro-1H-pyrazol-4-yl) methyl) phosphonates 6(a) according to general procedure C to afford the target compound as a yellow solid (0.95gr) with the following characteristic: Yield 70%, m p 176-178OC. IR (KBr cm-1) 3418-3384 (N-H), 3052 (stretching of Ar-H), 2940 and 2895 (CH3 & CH2 of aliphatic-CH), 1670 (C=O), 1478-1375 (stretching vibrations of pyrazolone ring), 1140 (C-O), 1245 (P=O), 1100 (C-F) and 745 cm-1(P-O). 1H-NMR (400MHz, DMSO-d6): ( PPM 1.32 (t, 6H, 2x P-O-CH2-CH3 J=7Hz), 1.94 (s, 3H, of CH3 group), 2.40 (d, 1H, CH of pyrazolone ring), 2.50 (t, 4H, -CH2-N-CH2-of morpholine ring J=7Hz), 3.65 (t, 4H, -CH2-O-CH2-of morpholine ring J=7Hz), 4.10 (q, 4H, 2x OCH2, J=7Hz), 4.27 (s, 2H, -N-CH2-N- of morpholine ring), 4.72 (s, 1H, P-C-H), 6.03 (s, 1H, NH) and 7.24-7.31 (m, 4H, of flourophenyl group). 13C-NMR (75 MHz, DMSO-d6): (PPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.3, 53.2, 66.4, 143.2, 118.9, 116.3 and 155.7 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11& C14, C12 & C13, C15, C16 & C20, C17 & C19 and C18 respectively.31P-NMR (161.89 MHz, DMSO-d6): (PPM. 21.11. m/z = 456.19 for C20H30FN4O5P. Anal. Found (Calcd) C: 51.83 (52.63), H: 6.12 (6.62), N: 11.67 (12.27), F: 3.56 (4.16), P: 6.09 (6.79).
Diethyl (((4 - chorophenyl) amino) (3 - methyl - 1 - (morpholinomethyl) -5-oxo-4, 5 - dihydro-1H-pyrazol-4-yl) methyl) phosphonates (6b) according to general procedure C to afford the target compound as a yellow solid (0.964gr) with the following characteristic: Yield 68%, m p 157-159°C. IR (KBr cm-1) 3420-3386 (N-H), 3055 (stretching of Ar-H), 2940 and 2895 (CH3 & CH2 of aliphatic-CH), 1675 (C=O), 1478-1375 (stretching vibrations of pyrazolone ring), 1254 (P=O), 1145 (C-O), 740 (P-O) and 730 cm-1 (C-Cl). 1H-NMR (400MHz, DMSO-d6): ( PPM 1.32 (t, 6H, 2x P-O-CH2-CH3 J=7Hz), 1.94 (s, 3H, of CH3 group), 2.40 (d, 1H, CH of pyrazolone ring), 2.50 (t, 4H, -CH2-N-CH2-of morpholine ring J=7Hz), 3.65 (t, 4H, -CH2-O-CH2-of morpholine ring J=7Hz), 4.10 (q, 4H, 2x OCH2, J=7Hz), 4.27 (s, 2H, -N-CH2-N- of morpholine ring), 4.65 (s, 1H, P-C-H), 5.95 (s, 1H, NH) and 7.02-7.25 (m, 4H, of chlorophenyl group). 13C-NMR (75 MHz, DMSO-d6): (PPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.3, 53.2, 66.4, 145.7, 114.9, 129.6 and 126.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11& C14, C12 & C13, C15, C16 & C20, C17 & C19 and C18 .respectively.31P-NMR (161.89 MHz, DMSO-d6): (PPM. 20.9. m/z = 472.16 for C20H30ClN4O5P. Anal. Found (Calcd) C: 50.00(50.80), H: 5.89 (6.39), N: 11.25 (11.85), Cl: 6.70 (7.50), P: 5.85 (6.55).
Diethyl (((4 - bromophenyl) amino) (3 - methyl-1- (morpholinomethyl) - 5 - oxo - 4, 5-dihydro-1H-pyrazol-4-yl) methyl) phosphonates 6(c) according to general procedure C to afford the target compound as a yellow solid (1.03gr) with the following characteristic: Yield 67%, m p 142-144°C. IR (KBr cm-1) 3420-3390 (N-H), 3065 (stretching of Ar-H), 2940 and 2895 (CH3 & CH2 of aliphatic-CH), 1680 (C=O), 1478-1375 (stretching vibrations of pyrazolone ring), 1259 (P=O), 1148 (C-O), 752 (P-O) and 650 cm-1 (C-Br). 1H-NMR (400MHz, DMSO-d6): ( PPM 1.32 (t, 6H, 2x P-O-CH2-CH3 J=7Hz), 1.94 (s, 3H, of CH3 group), 2.40 (d, 1H, CH of pyrazolone ring), 2.50 (t, 4H, -CH2-N-CH2-of morpholine ring J=7Hz), 3.65 (t, 4H, -CH2-O-CH2-of morpholine ring J=7Hz), 4.10 (q, 4H, 2x OCH2, J=7Hz), 4.27 (s, 2H, -N-CH2-N- of morpholine ring), 4.60 (s, 1H, P-C-H), 5.90 (s, 1H, NH) and 7.0-7.20 (m, 4H, of bromophenyl group). 13C-NMR (75 MHz, DMSO-d6): (PPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.3, 53.2, 66.4, , 146.6, 114.5, 132.4 and 115.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11& C14, C12 & C13, C15, C16 & C20, C17 & C19 and C18 .respectively.31P-NMR (161.89 MHz, DMSO-d6): (PPM. 19.90. m/z = 516.11 for C20H30BrN4O5P . Anal. Found (Calcd) C: 45.63 (46.43), H: 5.34 (5.84), N: 10.23 (10.83), Br: 14.84 (15.44), P: 4.79 (5.49).
Diethyl ((3 - methyl - 1 - (morpholinomethyl) - 5 - oxo - 4, 5-dihydro - 1H - pyrazol - 4 - yl) ((4-(trifluoromethyl) phenyl) amino) methyl) phosphonates 6(d) according to general procedure C to afford the target compound as a yellow solid (0.987gr) with the following characteristic: Yield 65%, m p 183-185OC. IR (KBr cm-1) 3422-3392 (N-H), 3067 (stretching of Ar-H), 2940 and 2895 (CH3 & CH2 of aliphatic-CH), 1673 (C=O), 1478-1375 (stretching vibrations of pyrazolone ring), 1256 (P=O), 1150 (C-O), 1110 (C-F) and 755 cm-1 (P-O). 1H-NMR (400MHz, DMSO-d6): ( PPM 1.32 (t, 6H, 2x P-O-CH2-CH3 J=7Hz), 1.94 (s, 3H, of CH3 group), 2.40 (d, 1H, CH of pyrazolone ring), 2.50 (t, 4H, -CH2-N-CH2 of morpholine ring J=7Hz), 3.65 (t, 4H, -CH2-O-CH2-of morpholine ring J=7Hz), 4.10 (q, 4H, 2x OCH2, J=7Hz), 4.27 (s, 2H, -N-CH2-N- of morpholine ring), 4.80 (s, 1H, P-C-H), 6.15 (s, 1H, NH) and 7.25-7.60 (m, 4H, of triflouromethylphenyl group).13C-NMR (75 MHz, DMSO-d6): (PPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.3, 53.2, 66.4, 150.9, 113.8, 125.9, 124.9 and 124.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11& C14, C12 & C13, C15, C16 & C20, C17 & C19, C18 and C21 respectively.31P-NMR (161.89 MHz, DMSO-d6): (PPM. 26.5. m/z = 506.19 for C21H30F3N4O5P. Anal. Found (Calcd) C: 49.00 (49.80), H: 5.47 (5.97), N: 10.46 (11.06), F: 10.65 (11.25), P: 5.42 (6.12).
Diethyl (((4-fluorophenyl) amino) (3-methyl-1- (4-methyl piperazin-1-yl) methyl) - 5 - oxo - 4, 5 - dihydro - 1H - pyrazol - 4 - yl) methyl) phosphonates 6(e) according to general procedure C to afford the target compound as a yellow solid (0.985gr) with the following characteristic: Yield 70%, m p 149-151OC. IR (KBr cm-1) 3418-3384 (N-H), 3052 (stretching of Ar-H), 2940 and 2895 (CH3 & CH2 of aliphatic-CH), 1674 (C=O), 1478-1375 (stretching vibrations of pyrazolone ring), 1254 (P=O), 1149 (C-O), 1108 (C-F) and 756 cm-1 (P-O). 1H-NMR (400MHz, DMSO-d6): ( PPM 1.32 (t, 6H, 2x P-O-CH2-CH3 J=7Hz), 1.94 (s, 3H, of CH3 group), 2.26 (s, 3H, CH3 attached to piperazin ring), 2.40 (d, 1H, CH of pyrazolone ring), 2.65 (m, 8H, (CH2)4 of piperazine ring), 4.10 (q, 4H, 2x OCH2, J=7Hz), 4.27 (s, 2H, -N-CH2-N- of morpholine ring), 4.72 (s, 1H, P-C-H), 6.03 (s, 1H, NH) and 7.24-7.31 (m, 4H, of flourophenyl group). 13C-NMR (75 MHz, DMSO-d6): (PPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.0, 52.2, 57.3, 46.6, 143.2, 118.9, 116.3 and 155.7 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11 & C14, C12 & C13, C15, C16, C17 & C21, C18 & C20 and C19.respectively.31P-NMR (161.89 MHz, DMSO-d6): (PPM. 20.10. m/z = 469.2 for C21H33FN5O4P. Anal. Found (Calcd) C: 52.92 (53.72), H: 6.58 (7.08), N: 14.32 (14.92), F: 3.45 (4.05), P: 6.00 (6.60).
Diethyl (((4 - chlorophenyl) amino) (3 - methyl - 1 - (4-methyl piperazin-1-yl) methyl) - 5 - oxo - 4, 5-dihydro-1H-pyrazol-4-yl) methyl) phosphonates 6(f) according to general procedure C to afford the target compound as a yellow solid (1.005gr) with the following characteristic: Yield 69%, m p 137-139°C. IR (KBr cm-1) 3420-3386 (N-H), 3055 (stretching of Ar-H), 2940 and 2895 (CH3 & CH2 of aliphatic-CH), 1676 (C=O), 1478-1375 (stretching vibrations of pyrazolone ring), 1256 (P=O), 1150 (C-O), 747 (P-O) and 735 cm-1 (C-Cl). 1H-NMR (400MHz, DMSO-d6): ( PPM 1.32 (t, 6H, 2x P-O-CH2-CH3 J=7Hz), 1.94 (s, 3H, of CH3 group), 2.26 (s, 3H, CH3 attached to piperazin ring), 2.40 (d, 1H, CH of pyrazolone ring), 2.65 (m, 8H, (CH2)4 of piperazine ring), 4.10 (q, 4H, 2x OCH2, J=7Hz), 4.27 (s, 2H, -N-CH2-N- of morpholine ring), 4.65 (s, 1H, NH), 5.95 (s, 1H, P-C-H) and 7.02-7.20 (m, 4H, of chlorophenyl group). 13C-NMR (75 MHz, DMSO-d6): (PPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.0, 52.2, 57.3, 46.6, 145.7, 114.9, 129.6 and 126.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11 & C14, C12 & C13, C15, C16, C17 & C21, C18 & C20 and C19..respectively.31P-NMR (161.89 MHz, DMSO-d6): (PPM. 19.80. m/z = 485.20 for C21H33ClN5O4P. Anal. Found (Calcd) C: 51.10(51.90), H: 6.34(6.84), N: 13.81 (14.41), Cl: 6.50 (7.30), P: 5.67 (6.37).
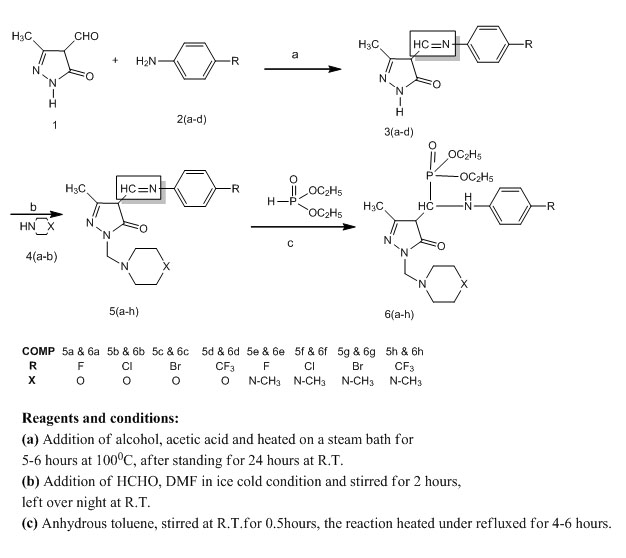 |
Scheme-1. Synthesis of Mannich bases of Pyrazole-5-one phosphonates 6(a-h) |
Diethyl (((4-bromophenyl) amino) (3 - methyl - 1 - (4-methyl piperazin-1-yl) methyl) - 5 - oxo - 4, 5 - dihydro-1H-pyrazol-4-yl) methyl) phosphonates 6(g) according to general procedure C to afford the target compound as a yellow solid (1.007gr) with the following characteristic: Yield 65%, m p 129-131°C. IR (KBr cm-1) 3422-3392 (N-H), 3065 (stretching of Ar-H), 2940 and 2895 (CH3 & CH2 of aliphatic-CH), 1678 (C=O), 1478-1375 (stretching vibrations of pyrazolone ring), 1254 (P=O), 1152 (C-O), 743 (P-O) and 655 cm-1 (C-Br). 1H-NMR (400MHz, DMSO-d6): ( PPM 1.32 (t, 6H, 2x P-O-CH2-CH3 J=7Hz), 1.94 (s, 3H, of CH3 group), 2.26 (s, 3H, CH3 attached to piperazin ring), 2.40 (d, 1H, CH of pyrazolone ring), 2.65 (m, 8H, (CH2)4 of piperazine ring), 4.10 (q, 4H, 2x OCH2, J=7Hz), 4.27 (s, 2H, -N-CH2-N- of morpholine ring), 4.65 (s, 1H, NH), 5.95 (s, 1H, P-C-H) and 7.0-7.20 (m, 4H, of bromophenyl group). 13C-NMR (75 MHz, DMSO-d6): (PPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.0, 52.2, 57.3, 46.6, 146.6, 114.5, 132.4 and 115.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11 & C14, C12 & C13, C15, C16, C17 & C21, C18 & C20 and C19 respectively.31P-NMR (161.89 MHz, DMSO-d6): (PPM. 19.60. m/z = 529.15 for C21H33BrN5O4P. Anal. Found (Calcd) C: 46.75 (47.55), H: 5.77 (6.27), N: 13.60 (13.20), Br: 14.40 (15.00), P: 5.14 (5.84).
Diethyl ((3-methyl - 1 - (4 -methyl piperazin - 1 - yl) -methyl - 5 - oxo - 4, 5 -dihydro-1H-pyrazol - 4 - yl) ((4 - (trifluoromethyl) phenyl) amino) methyl) phosphonates 6(h) according to general procedure C to afford the target compound as a yellow solid (1.168gr) with the following characteristic: Yield 75%, m p 167-169°C. IR (KBr cm-1) 3422-3392 (N-H), 3067 (stretching of Ar-H), 2940 and 2895 (CH3 & CH2 of aliphatic-CH), 1680 (C=O), 1478-1375 (stretching vibrations of pyrazolone ring), 1254 (P=O), 1115 (C-O), 1108 (C-F) and 756 cm-1 (P-O). 1H-NMR (400MHz, DMSO-d6): ( PPM 1.32 (t, 6H, 2x P-O-CH2-CH3 J=7Hz), 1.94 (s, 3H, of CH3 group), 2.26 (s, 3H, CH3 attached to piperazin ring), 2.40 (d, 1H, CH of pyrazolone ring), 2.65 (m, 8H, (CH2)4 of piperazine ring), 4.10 (q, 4H, 2x OCH2, J=7Hz), 4.27 (s, 2H, -N-CH2-N- of morpholine ring), 4.65 (s, 1H, NH), 5.95 (s, 1H, P-C-H) and 7.25-7.60 (m, 4H, of triflouromethyl phenyl group). 13C-NMR (75 MHz, DMSO-d6): (PPM. 155.6, 33.7, 172.0, 19.3, 57.5, 62.2, 16.3, 70.0, 52.2, 57.3, 46.6, 150.9, 113.8, 125.9, 124.9 and 124.1 corresponding to C1, C2, C3, C4, C5, C6 & C8, C7 & C9, C10, C11 & C14, C12 & C13, C15, C16, C17 & C21, C18 & C20, C19 and C22 .respectively. 31P-NMR (161.89 MHz, DMSO-d6): (PPM. 24.5. m/z = 519.22 for C22H33F3N5O4P. Anal. Found (Calcd) C: 50.06 (50.86), H: 5.90 (6.40), N: 12.78 (13.48), F: 10.37 (10.97), P: 5.26 (5.96).
Biological Activity
The antimicrobial activity [13] of these newly synthesized compounds was performed according to disc diffusion method, as recommended by the National Committee for Clinical Laboratory. The synthesized compounds were used at the concentration of 250μg/ml DMF as a solvent [14].
Anti-Bacterial Activity
The antibacterial activity of Mannich bases containing Pyrazole-5-one Phosphonates 6(a-h) were screened against the Staphylococcus aureus and Bacillus cerus (gram positive) and Escherichia coli, Pseudomonasaeruginosa (gram negative) organisms. Most of the compounds exhibit good antibacterial activity against both bacteria. The presence of fluoro (6d, 6h) and chloro (6b, 6f) were showed more activity than other substituted compounds. Here Amoxicillin is tested as reference compound to compare the activity. The anti-bacterial activity was shown in the Table 1.1
Antifungal activity (Diameter zone of Inhibition in mm) Compounds of 6(a-h).
| COMP NO | Zone of inhibition(mm) (250 µg/ disc) | |||
|---|---|---|---|---|
|
Staphylococcus aureus NCCS2 079 |
Bacillus cereus NCCS 2106 |
Escherichia coli NCCS 2065 |
Pseudomonas aeruginosa NCCS 2200 |
|
| 6a | 12 | 14 | 11 | 13 |
| 6b | 13 | 15 | 12 | 14 |
| 6c | 10 | 12 | 09 | 11 |
| 6d | 16 | 18 | 15 | 17 |
| 6e | 10 | 12 | 09 | 11 |
| 6f | 12 | 14 | 11 | 13 |
| 6g | 08 | 10 | 07 | 09 |
| 6h | 14 | 16 | 13 | 15 |
| Amoxicillin | 21 | 27 | 24 | 22 |
Anti-Fungal Activity
The anti-fungal activity of Mannich bases containing Pyrazole-5-one Phosphonates 6(a-h) was screened against the Aspergillus niger and Candida albicans. Most of the compounds exhibit good antifungal activity against both fungai. The presence of fluoro (6d, 6h) and chloro (6b, 6f) were showed more activity than other substituted compounds. Here Ketoconazole is tested as reference compound to compare the activity. The anti-fungal activity was shown in the Table 1.2.
Antifungal activity (Diameter zone of Inhibition in mm) Compounds of 6(a-h).
| COMP NO | Zone of inhibition(mm) (250 µg/ disc) | ||
|---|---|---|---|
|
Aspergillus niger NCCS 1196 |
Candida albicans NCCS 3471 |
||
| 6a | 11 | 13 | |
| 6b | 14 | 16 | |
| 6c | 10 | 12 | |
| 6d | 17 | 19 | |
| 6e | 09 | 11 | |
| 6f | 12 | 14 | |
| 6g | 08 | 10 | |
| 6h | 14 | 16 | |
| Ketoconazole | 22 | 25 | |
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The author V. Esther Rani thanks to U G C - S A P and U G C - B S R, New Delhi for financial assistance. They are also thankful to IICT Hyderabad and CDRI Lucknow for spectral and analytical data.














