RESEARCH ARTICLE
Anticonvulsant Activities of 4-benzylidene-6-(4-methyl-phenyl)-4,5-dihydropyridazin-(2H)-ones and 4-benzylidene-6-(4-chloro-phenyl)-4,5-dihydropyridazin-(2H)-ones
Mohammad Asif1, *, Anita Singh2
Article Information
Identifiers and Pagination:
Year: 2016Volume: 3
First Page: 203
Last Page: 214
Publisher Id: PHARMSCI-3-203
DOI: 10.2174/1874844901603010196
Article History:
Received Date: 16/11/2015Revision Received Date: 11/07/2016
Acceptance Date: 18/07/2016
Electronic publication date: 27/10/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Background:
Two series of 4-benzylidene-6-(4-methyl-phenyl)-4,5-dihydropyridazin-(2H)-one compounds (3a-e) and 4-benzylidene-6-(4-chloro-phenyl)-4,5-dihydropyridazin-(2H)-ones (3f-j) were synthesized and evaluated as anticonvulsant agents.
Methods:
Synthesized compounds (3a-3j) were tested against maximum electro shock (MES) and Isoniazid (INH) induced convulsion methods for anticonvulsant activities and neurotoxicity.
Results:
In MES induced convulsions, result found that the compounds 3e and 3j exhibited highest anticonvulsant activities. In INH induced convulsions, result was indicated that all the compounds exhibited good anticonvulsant activities., whereas compounds 3d and 3j showed maximum activity. Methyl derivatives were more active than chloro derivatives. Phenytoin sodium (25mg/kg) and sodium vaproate (50mg/kg) were used as reference drugs. All these synthesized pyridazinone compounds (3a-j) did not exhibit any neurotoxicity up to 100 mg/kg dose levels.
Conclusion:
All compounds showed good anticonvulsant activities against both MES and INH induced convulsion models. Many such explorations are anticipated in the near future.
INTRODUCTION
Pyridazine and pyridazinone derivatives having various types of pharmacological activities such as antimicrobial, antitubercular, antifungal, antiviral, anti-HIV, analgesic, anti-inflammatory, antipyretic, selective COX-2 inhibitors, insecticidal, molluscicidal, insect and their larval growth regulator, anticancer, anti-asthmatic, anti-allergic, antifeedant, herbicidal, antidiabetic, anti-ulcer, caridovascular agent like PDE-inhibitors, antihypertensive, antiplatelet, ionotropic, antianginal, antiarrthmic, and neurological activity, like anti anxiety, antidepressant, anticonvulsant and other anticipated biological properties. It is also used as intermediate for drugs synthesis and agrochemicals [1-15].
Epilepsy is supposed to be a condition of paroxysmal cerebral dysrhythmia. A common type of epilepsy is grand mal (Generalized tonic-clonic) and petit mal (absence seizures) epilepsy. Grand mal epilepsy: Major epilepsy (Generalized tonic-clonic) commonest last to 1-2 min and illustrated by complete loss of consciousness, followed by transient muscular rigidity (tonic phase) and ultimately falls into violent clonic convulsions followed by prolonged sleep and depression of all “CNS” function. Petit mal: It this usually momentary loss of consciousness prevails. This particular state is free of convulsions. However, occasionally blinking movements of the eyelids and jerking movements of the head or arms are observed [16-18]. Epilepsy management is still a major problem due to uncontrolled seizures in some types of epilepsy, drug toxicity, side effects and long period treatment even throughout a life time. Risks of tolerance developed against the presently used medicines. Various antiepileptic drugs available in the market, due to above mentioned reasons, development of new antiepileptic drugs are still necessary. The maximum electroshock (MES) induced seizures in animal represents the grand mal type of epilepsy and chemo convulsions are due to Pentylenetetrazole (PTZ), strychnine (STR), isoniazide (INH) which produced petit mal type of convulsions [16-20]. The drug design is based on the opinion that at least one phenyl or aryl group in close proximity to two electron donor atoms in the molecule is necessary for the activity against MES induced seizure, an alkyl group substituted close to two electron donor atoms in the molecule is necessary for the activity against chemo convulsions. Many researches indicated that the presence of at least one aryl group, one or two electron donor atoms and/or an imino (NH) group in spatial arrangement is vital for antiepileptic activity. So, syntheses of new pyridazinone compounds are tested for their anticonvulsant screening. Most of the drugs are used as antiepileptic agents today contain ureide structure such as barbiturates, benzodiazepines, oxazolidinediones, succinimides and glutarimides [21-25].
Thus, the present work is to concentrate on preparation of various new substituted pyridazinones and tested for their anticonvulsant activity against MES and INH-induced convulsions. The essential requirement is not only design and development of the drug molecule, but also the progress of its testing methods, mode of actions and its usefulness. New pyridazinone derivatives are designed to prepare effective drug molecule with minimum or lesser side effects.
CHEMISTRY
All compounds were synthesized according to Schemes (1). All chemicals were used for synthesis of pyridazinone compounds were purchased from Loba Chem, Central drug house (CDH), Merck, SD-fine and High media, India. Melting points of all the synthesized compounds were determined by open tube capillary method and were uncorrected. The purity of the compounds was checked by using thin layer chromatography (TLC) methods and plates were prepared from silica gel G and solvent system toulene, ethylacetoacetae, formic acid (4:5:1 ratio) and benzene, acetone (3:1) was used as mobile phase, the spot of synthesized compounds were visualized by using the iodine vapors and ultra-violet (UV) light. Elementary analyses were carried out on a LECO CHNS 932 analyzer for determining C,H,N atom values. The FT-IR spectra were recorded on Bio-rad FTS-135 spectrophotometer using KBr pellets; λ max values are given in cm-1. The NMR spectra were recorded on Bruker Avance-II 400 NMR spectrometer using CDCl3 as solvent and tetra methyl silane (TMS) was used as an internal standard and chemical shifts were given in δ (ppm) scale and the Mass spectra were recorded on JEOL-JMS-DX 303 system, prepared with direct inlet probe system.
SYNTHESIS OF AROYL-PROPIONIC ACID DERIVATIVES (1A AND 1B)
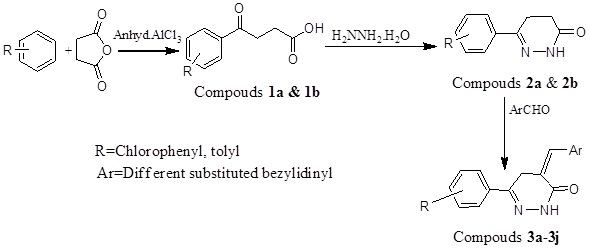
Anhydrous AlCl3 (0.15 mol) was suspended in appropriate aryl hydrocarbons (50 mL) under the anhydrous condition and the reaction mixture was refluxed on a water bath. Then the succinic anhydride (0.10 mol) was added into the reaction mixture in small portions with nonstop stirring for 6 h (Scheme 1). The reaction mixture was cooled down, left overnight and after that made acidic by adding an ice cold concentrated HCl solution (2.5% v/v). The reaction mixture was concentrated to a small volume by heating on a water bath. The precipitate was formed and separated out and collected it. The precipitated product was purified by dissolving in 5% w/v Na2CO3 solution and followed by extraction with ether. The acidification with dilute HCl acid gave an aroyl propionic acid (Fig. 1) in the aqueous layer [26-28].
Other propionic acids were synthesized by a similar process with minor changes in temperature of reaction and nitrobenzene used as a solvent. All these compounds were re-crystallized with aqueous ethanol.
β-toloyl Propionic Acid (1a)
Molecular formula: C11H12O3, molecular weight: 192, melting point: 122°C; 67% yield. IR: (KBr, cm-1): 1658.80 (CO), 2995.62 (CH), 3412.60 (OH), 1H-NMR (DMSO-d6, δ ppm): 2.38 (s, 3H, CH3), 2.78 (t, 2H, CH2), 2.99 (t, 2H, CH2), 7.26 (t, 2H, H3',5', Ar-H), 12.15 (s,1H, COOH), MS (m/z): 193 (M++1).
 |
Scheme 1. Synthetic route of 6-aryl-4-benzylidene-4,5-dihydro pyridazino-3(2H)-ones (3a-j). |
4-Chloro Benzoyl Propanoic Acid (1b)
Molecular formula C10H9O3Cl, molecular weight 212, melting point: 122 0C, yield 54. IR Spectra: 2950 cm-1 (CH), 3212.60 (OH), 1710 cm-1 (C=O).NMR Spectra: 1HNMR(CDCl3) ppm 2.82 (2H, t, CH2), 3.31 (2H, t, CH2), 7.74 (CH2, m, H-3, 5), 7.79 (2H, m, H-2, 6), MS (m/z): 112 (M+1).
SYNTHESIS OF 6-ARYL-4,5-DIHYDROPYRIDAZIN-3(2H)-ONE DERIVATIVES (2A AND 2B)
To a solution of β-aroyl propionic acid (1a) (0.1 mol) in CH3OH (30 ml), NH2NH2. H2O (1ml) and CH3COONa (0.5 g) were added and the reaction mixture was refluxed for 6 h. After completing the reaction, CH3OH was distilled off and reaction mixture was transferred into cold water [29-31]. The solid product (Fig. 1) was separated out, filters it and re-crystallized from methanol (Scheme 1).
Other pyridazinone was synthesized by a similar process with minor alteration in temperature of reactions and solvent nitrobenzene is used.
6-(4-methylphenyl)-4,5-dihydropyridazin-3(2H)-one (2a)
Molecular formula C11H12N2O, molecular weight 188.20, melting point: 151 0C, yield: 71%, Rf value 0.70, IR: (KBr,cm-1): 1658.7 (C=O), 3217.4 (NH), 1510.8 (C=N), 13C NMR (CDCl3) (ppm): 20.9, 27.1, 35.6, 128.2, 129.3, 128.9, 140.0, 155.6, 161.2, 1H NMR (CDCl3) (ppm): 2.38 (s, 3H, CH3), 2.61 (m,2H, CH2-CO), 2.97 (t, 2H, CH2-aryl), 7.26 (t, 2H, Ar-H3',H5'), 7.63 (d, 2H, Ar-H2',H6'), 8.79 (s, 1H, NH), MS (m/z): 189 (M+1).
6-(4-chlorophenyl)-4,5-dihydropyridazin-3(2H)-one (2b)
Molecular formula C10H9ClN2O, molecular weight 208, yield 55%, melting point. 130 0C, Rf value 0.60. IR (cm-1)1685(C=O), 1352 (NO2), 3000 (CH), 3550 (NH), 2.60 (t, 2H, CH2), 3.2 (t, 2H, CH2), 7.53-7.68 (m, 2H, H-3’-H-5’, Ar-H), 7.97 (d, 2H, H-2’-H-6’, Ar-H), 8.78 (s, NH).
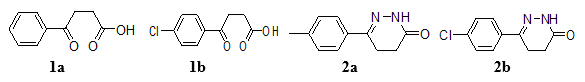 |
Fig. (1). Structures of aroyl-propionic acids (1a-b) and 6-arylpropyridazine derivatives (2a-b). |
SYNTHESIS OF 4-BENZYLIDENE-6-(4-SUBSTITUTED-ARYL)-4,5-DIHYDROPYRIDAZIN-(2H)-ONES
The 6-(4-chlorophenyl)-4,5-dihydropyridazin-3(2H)-one or 6-(4-methylphenyl)-4,5-dihydro pyridazin-3(2H)-one (0.005 mol), appropriate aldehyde derivatives (0.005 mol) in C2H5OH (20 ml) and ethanolic C2H5ONa solution were added. Then reaction mixture was left overnight at room temperature, diluted with water and rendered acidic with concentrated HCl and filter the product. The crude product (3a-j) (Fig. 2) was re-crystallized with 90% ethanol [32-34].
4-benzylidene-6-(4-chloro-phenyl)-4,5-dihydropyridazin-(2H)-one (3a)
Molecular formula C17H13ClN2O, molecular weight 296, yield: 62%; melting point; 170 ºC; IR (KBr) in cm-1: KBr): 3185 (NH), 2949 (Ar-CH), 2831 (C-H), 1602.56 (-C=C), 1255.3 (C-N), 1685 (C=O), 718 (C-Cl), 1H-NMR (CDCl3, δ, ppm): 3.9 (s, 2H, CH2), 7.16 (s, 1H, H-4), 7.14-7.48 (m, 5H, aryl ring), 7.20 (s, 1H, CH), 7.42 and 7.73 (d, each, p-substituted aryl ring), 10.72 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 28.4, 126.2, 127.7, 128.4, 129.0, 129.2, 129.3, 130.4, 134.2, 134.9, 136.1, 155.6, 160.0, MS (m/z): 297 (M++1). Elemental analysis: calculated (%): C = 68.81, H = 4.42, N = 9.44; Found (%): C= 68.93, H = 4.45, N = 9.43.
4-(4-chloro-benzylidene)-6-(4-chloro-phenyl)-4,5-dihydropyridazin-(2H)-one (3b)
Molecular formula C17H12Cl2N2O, molecular weight 331, yield: 55%; melting point; 190 ºC; IR (cm-1, KBr): 3179 (NH), 2957 (C-H), 1688 (C=O), 726 (C-Cl), 1602.56 (C=C), 1292.07 (C-N), 1H-NMR (CDCl3, δ, ppm): 3.9 (s, 2H, CH2), 7.20 (s, 1H, CH), 8.6 (1H, s, NH), 7.11 (s, 1H, H-4), 7.23 (m, 4H, H-2,3,5,6, aryl ring), 7.34 and 7.41 (d, each, p-substituted aryl), 10.97 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 28.4, 127.6, 128.8, 129.0, 129.2, 129.3, 130.4, 136.1, 133.0, 134.2, 155.6, 162.2, MS (m/z): 332 (M++1). Elemental analysis: calculation (%): C= 61.65, H=3.65, N= 8.46; Found (%): C= 61.55, H= 3.67, N = 8.47.
4-(4-hydroxy-benzylidene)-6-(4-chloro-phenyl)-4,5-dihydropyridazin-(2H)-one (3c)
Molecular formula C17H13ClN2O2, molecular weight Molecular Weight: 312, yield: 52%, melting point; 180 ºC; IR (cm-1, KBr): 3175 (NH), 2942 (CH), 1688 (C=O), 722 (C-Cl), 1H-NMR (CDCl3, δ, ppm): 3.84 (s, 2H, CH2), 6.15 (m, 1H, OH), 7.06 (m, 2H, H-2,6, benzyl ring), 7.04 (s, 1H, CH), 7.31 (s, 1H, H-4), 7.47 and 7.71 (d, each, p-substituted aryl), 7.49 (m, 2H, H-3,5, aryl ring), 9.41 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 28.4, 115.6, 127.5, 127.6, 129.0, 129.2, 129.3, 130.4, 134.2, 136.1, 156.5, 155.6, 162.0, MS (m/z): 313(M++1). Elemental analysis: calculated (%): C= 65.29, H= 4.19, N= 8.96; Found (%): C= 65.39, H= 4.17, N= 8.97.
4-(4-methoxy-benzylidene)-6-(4-chloro-phenyl)-4,5-dihydropyridazin-(2H)-one (3d)
Molecular formula C18H15ClN2O2, molecular weight 326, Yield: 67%; melting point; 155 ºC; IR (KBr) in cm-1: 3165 (NH), 3001 (CH), 1675 (C=O), 1602.57 (C=C), 1255.30 (C-N), 1166.72 (OCH3), 717 (C-Cl), 1H-NMR (CDCl3, δ, ppm): 7.20 (s, 2H, CH2), 3.70 (s, 2H, CH2), 3.71 (s, 3H, OCH3), 6.8 (s, 1H, H-4), 7.13 and 7.36 (d, each, p-substituted benzyl ring), 7.59 (m, 2H, H-3,5, phenyl ring), 7.68 (m, 2H, H-2,4, phenyl ring), 10.70 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 28.4, 115.6, 127.5, 127.6, 129.0, 129.2, 129.3, 130.4, 134.2, 136.1, 156.5, 155.6, 162.0, MS (m/z): 326(M+). Elemental analysis: calculated (%): C= 66.16, H= 4.63, N= 8.57; Found (%): C= 66.23, H= 4.61, N= 8.55.
4-(4-hydroxy-3-methoxy-benzylidene)-6-(4-chloro-phenyl)-4,5-dihydro-pyridazin-(2H)-one (3e)
Molecular formula C18H15ClN2O3, molecular weight 342, yield: 48%; melting point; 201 ºC; IR (cm-1, KBr): 3173 (NH), 2951 (CH), 1680 (C=O), 713 (C-Cl), 1H-NMR (CDCl3, δ, ppm): 3.78 (s, 2H, CH2), 3.72 (s, 3H, OCH3), 6.53 (s, 1H, H-4), 7.07-7.26 (m, 3H, H-2,5,6, di-substituted aryl ring), 7.2 (s, 2H, CH2), 7.46 and 7.71 (d, each, p-substituted aryl ring), 10.92 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 28.4, 56.3, 113.2, 116.6, 119.9, 128.5, 129.0, 129.2, 129.3, 130.4, 134.2, 136.1, 142.1, 149.1, 155.6, 162.1,162.2, MS (m/z): 343(M++1). Elemental analysis: calculated (%): C = 63.07, H=4.41, N= 8.17; Found (%): C= 62.97, H= 4.39, N= 8.19.
4-benzylidene-6-(4-Methyl-phenyl)-4,5-dihydropyridazin-(2H)-one (3f)
Molecular formula C18H16N2O, molecular weight 276, yield: 55%; melting point; 160 ºC; IR (cm-1, KBr): 3180 (NH), 2995 (CH), 1696 (C=O), 1H-NMR (CDCl3, δ, ppm): 3.94 (s, 3H, CH3), 3.02 (s, 2H, CH2), 6.86 (s, 1H, H-4), 7.02-7.43 (m, 5H, benzyl ring), 7.22 (s, 1H, CH), 7.46 and 7.79 (d, each, p-substituted aryl ring), 8.92 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 20.9, 28.4, 126.2, 127.7, 128.2, 128.4, 128.9, 129.2, 129.3, 134.2, 134.9, 140.0, 155.6, 162.2, MS (m/z): 277 (M++1). Elemental analysis: calculated (%): C= 78.24, H= 5.84, N= 10.14; Found (%): C= 78.23, H= 5.87, N= 10.17.
4-(4-chloro-benzylidene)-6-(4-methyl-phenyl)-4,5-dihydropyridazin-(2H)-one (3g)
Molecular formula C18H15ClN2O, molecular weight 310, yield: 48%; melting point; 175 ºC; IR (cm-1, KBr): 3173 (NH), 2939 (CH), 1683 (C=O), 718 (C-Cl), 1H-NMR (CDCl3, δ, ppm): 3.90 (s, 3H, CH3), 2.6 (s, 2H, CH2), 7.23 (s, 1H, CH), 7.24 (s, 1H, H-4), 7.26 and 7.56 (d, each, p-substituted aryl ring), 7.28-7.35 (m, 4H, H-2,3,5,6, phenyl ring), 10.69 (s, 1H, NH), 7.26-7.56 (m, H, 4-CH3-benzyl ring), 7.28-7.35 (m, 4H, 4-chlorophenyl ring), 10.69 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 20.9, 28.4, 127.6, 128.2, 128.8, 128.9, 129.2, 129.3, 133.0, 134.2, 140.0, 155.6, 162.2, MS (m/z): 311 (M++1). Elemental analysis: calculated (%): C= 69.57, H=4.86, N= 9.01; Found (%): C= 69.53, H= 4.87, N= 8.99.
4-(4-hydroxy-benzylidene)-6-(4-methyl-phenyl)-4,5-dihydropyridazin-(2H)-one (3h)
Molecular formula C18H16N2O2, molecular weight 292, yield: 47%; melting point; 185 ºC; IR (cm-1, KBr): 3165 (NH), 2957 (CH), 1695 (C=O), 1H-NMR (CDCl3, δ, ppm): 3.93 (s, 3H, CH3), 2.82 (s, 2H, CH2), 6.41 (s, 1H, H-4), 6.63 (m, 2H, H-2,6, phenyl ring), 6.66 (m, 2H, H-2,6, benzyl ring), 6.79 (m, 2H, H-3,5, phenyl ring), 6.81 (m, 2H, H-3,5, benzyl ring), 7.20 (s, 1H, CH), 12.25 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 20.9, 28.4, 115.6, 127.5, 127.6, 128.2, 128.9, 129.2, 129.3 134.2, 140.0, 155.6, 156.5, 162.0, MS (m/z): 293 (M++1). Elemental analysis: calculated (%): C = 73.96, H= 5.52, N= 9.58; Found (%): C= 73.98, H= 5.51, N= 9.57.
4-(4-methoxy-benzylidene)-6-(4-methyl-phenyl)-4,5-dihydropyridazin-(2H)-one (3i)
Molecular formula C19H18N2O2, molecular weight 306, yield: 46%; melting point; 202 ºC; IR (cm-1, KBr): 3185 (NH), 2954 (CH), 1686 (C=O), 1H-NMR (CDCl3, δ, ppm): 3.83 (s, 3H, CH3), 3.04 (s, 3H, CH2), 3.92 (s, 2H, OCH3), 6.79 (s, 1H, H-4), 7.01 (s, 1H, CH), 7.37 and 7.82 (d, each, p-substituted aryl ring), 7.49 (m, 2H, H-3,5, phenyl ring), 7.72 (m, 2H, H-2,6, phenyl ring), 11.15 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 20.9, 28.4, 56.0, 114.0, 127.2, 128.2, 128.9, 129.2, 129.3, 134.2, 140.0, 155.6, 162.1, 162.2, MS (m/z): 307 (M+). Elemental analysis: calculated (%): C= 74.49, H= 5.92, N= 9.14; Found (%): C= 74.53, H= 5.91, N= 9.13.
4-(4-hydroxy-3-methoxy-benzylidene)-6-(4-methyl-phenyl)-4,5-dihydropyridazin-(2H)-one (3j)
Molecular formula C19H18N2O3, molecular weight 322, yield: 51%; melting point; 175 ºC; IR (cm-1, KBr): 3189 (NH), 2948 (CH), 1687 (C=O), 1H-NMR (CDCl3, δ, ppm):) 3.86 (s, 2H, CH2), 3.25 (s, 3H, OCH3), 2.39 (s, 3H, CH3), 6.34 (s, 1H, OH), 6.82 (s, 1H, H-4), 7.04 (m, 1H, H-6, benzyl ring), 7.24 (s, 1H, CH), 7.25 (m, 2H, H-3,5, phenyl ring), 7.53 (m, 1H, H-2, benzyl ring), 7.68 (m, 2H, H-2,6, phenyl ring), 7.75 (m, 1H, H-5, benzyl ring), 10.73 (s, 1H, NH), 13C NMR (CDCl3) (ppm): 20.9, 28.4, 56.3, 113.2, 155.6, 116.6, 119.9, 128.2, 128.5, 128.9, 129.2, 129.3, 134.2, 140.0, 142.1, 149.1, 162.2,MS (m/z): 323 (M++1). Elemental analysis: calculated (%): C=70.79, H= 5.63, N= 8.69; Found (%): C= 70.83, H= 5.65, N= 8.67.
BIOLOGICAL EVALUATION
The synthesized compounds were subjected to the following evaluation. All chemicals were purchased from CDH (Central drug House), Merck, Loba Chem, and S.D. Fine Chemicals, Mumbai, India and used without purification. Strychnine (STR) (Sigma Aldrich, India) and Phenytoin (Sun Pharma Ltd, India) were used in the present study.
Experimental Animals
Swiss albino rats weighing 25-30 g were maintained under controlled conditions of light (12 hr) and temperature 25±2°C in the animal house of Department of Pharmacy, GRD(PG)IMT, Dehradun, India, two weeks before the experiment for acclimatization. Animals had access to food and water ad libitum. All pharmacological activities were carried out as per Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) norms (Regn No: 1145/a/07/CPCSEA), after getting the approval from the Institutional Animal Ethics Committee (IAEC) of Department of Pharmacy, GRD (PG) IMT, Dehradun, India.
Neurotoxicity Screening
Minimal motor impairment was tested on mice by using the rotarod (Techno, India) test model. The mice were skilled to stay on an accelerating rotarod (diameter 3.2 cm) that rotates at 10 revolutions per min (rpm). Before skilled mice were given test compounds i.p. in doses of 25, 50 and 100 mg/kg. Neurotoxicity was signified by the incapability of the animal to keep equilibrium on the rod for at least one min. in each of the three tests [35].
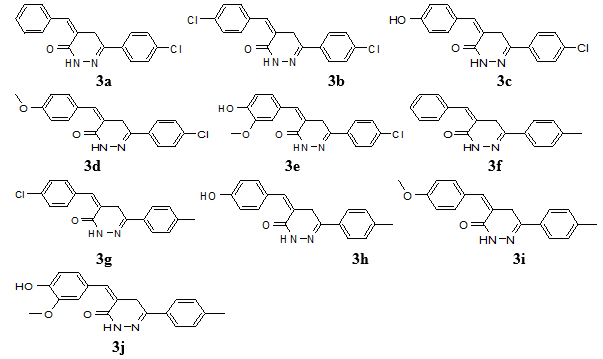 |
Fig. (2). Structures of 4-benzylidene-6-(4-substituted-/3-nitro-phenyl)-4,5-dihydro pyridazin-(2H)-ones (3a-j). |
Anticonvulsant Activities
Male albino mice were used to test the synthesized pyridazinone derivatives. Maximal Electro Shock (MES) and Isoniazid (INH) induced seizure methods were used [35-37].
Maximal Electro Shock (MES)-induced Convulsion Method
The MES method (Model: Techno Electro Convulso-meter) was used for inducing seizures. The MES induced seizures in mice correspond to grand-mal type of epilepsy. In MES, electro shock was applied to the ear pinna by passing 50 mA current for 0.2 sec. The MES seizures were divided into five stages (a) flexion (b) extensor (c) clonus (d) stupor and (e) recovery or death. Suspension of test compounds were formed in 0.5% carboxyl methyl cellulose (CMC) and were given intraperitoneally (i.p.) at dose level 25 mg/Kg body weight to different groups of mice and each group having five mice. Reduction in the extension phase was calculated and compared to phenytoin sodium, which was used as a standard at 25mg/Kg body weight dose. The animals were examined for all the five stages. The abolition of the extensor phase in drug treated groups was taken as the experimental standard for anticonvulsant activity.
Isoniazid (INH) Induced Seizures Method
Isoniazide (INH) induced seizures method was carried out on mice (25-30 g). The mice were injected with test drugs 50 mg/kg i.p. After 30 min, INH injection of dose 250 mg/kg was given i.p. The series of seizure latency, seizure time and percentage protection were studied. Animals displaying these seizure patterns were detected. The reference drugs used in this model was phenytoin sodium (25mg/kg) and sodium valproate (100mg/kg).
Statistical Evaluation
All the values were determined as mean ± S.E.M. Statistical analysis was obtained by using one-way analysis of variance (ANOVA). If the overall p-Value was found statistically significant (p< 0.05), further comparisons between groups were arranged according to Tukey’s test.
RESULTS
Chemistry
All synthesized pyridazinone compounds were characterized by spectral analysis via IR, NMR, mass spectra and elemental analysis data. The IR spectrum exhibited the peak at 1700, 3450, and 1580 cm-1 validated the occurrence of C=O, NH and C=C groups. The 1HNMR spectrum proved the signal in the triplet near δ=2.8 for CH2 protons at 5-positions, another triplet is viewed at about δ=3.0 for CH2 at 4 positions. Aromatic protons were detected in the region from δ=7.0-8.0, mass of compounds determined by mass spectroscopy. Elementary analyses gave satisfactory results and the values were found to be in range of ±0.4% theoretical value for the element analyzed or calculated (C,H,N). The presences of substitutes were determined in the IR, and 1HNMR spectra according to the assigned value. In the 1HNMR the signals of the respective protons of the prepared 3(2H)-pyridazinone compounds were verified on the basis of their chemical shifts and multiplicities. The other compounds are also identified in a similar manner. Elemental analyses gave satisfactory results.
Neurotoxicity
All the synthesized pyridazinone compounds (3a-j) did not exhibit any neurotoxicity up to 100 mg/kg dose levels. Neurotoxicity of compounds was pointed out by the lack of ability of the animal to retain equilibrium on the rod for at least one minute in each of the three trials.
ANTICONVULSANT ACTIVITIES
Anticonvulsant Activity Against MES-induced Convulsions
Anticonvulsant activities (ACAs) of aryl-4,5-dihydropyridazinones against maximal electro shock (MES) were found significant ACA when compared with the control group. Two series of 6-(4-Cl-Phenyl)-4-arylidene-4,5-dihydropyridazin-3(2H)-one (3a-e), 6-(4-CH3-Phenyl)-4-arylidene-4,5-dihydropyridazin-3(2H)-one derivatives (3f-j) were evaluated as anticonvulsant agents against MES-induced convulsion method. Result found that the compounds 3e and 3j exhibited highest ACA, these compounds having 6-methyl phenyl, 6-chloro-phenyl pyridazinone derivatives and act by reducing extensor phase. Methyl derivatives were more active than chloro derivatives (Table 1). For ACAs PHT sod. (25mg/kg) and sod. valproate (VPA) (50mg/kg) was used as reference drugs. Results indicated that synthesized pyridazinone compounds have moderate to good anticonvulsant activity.
|
S. No. |
Groups | Flexion (sec) |
Extensor (sec) |
Clonus (sec) |
Stupor (sec) |
Recovery (%) |
|---|---|---|---|---|---|---|
| 1 | Control | 3.34 ± 0.125 | 15.76±01.11 | 6.76 ± 01.11 | 48.90±07.65 | 60 |
| 2 | PHT Sod | 1.44 ± 0.051a | 0.00 ±0.00a | 1.84 ±0.23 | 7.78 ±0.58a | 100 |
| 3 | Sod VPA | 3.24±0.82 | 5.36±0.68 | 7.59±1.24 | 46.43±2.43 | 80 |
| 4 | Compd3a | 2.0±0.12aγ | 6.26±1.45aγ | 3.62±1.12aγ | 20.20±3.50aγ | 80 |
| 5 | Compd3b | 2.11±0.09aγ | 7.21±1.63aγ | 4.24±1.42bγ | 18.32±3.48aγ | 80 |
| 6 | Compd3c | 2.21±0.40aγ | 6.63±1.72aγ | 3.88±1.64aγ | 18.50±2.34aγ | 100 |
| 7 | Compd3d | 1.92±0.28aγ | 6.24±0.54aγ | 4.12±2.12bγ | 16.64±1.90aγ | 100 |
| 8 | Compd3e | 1.84±0.36aγ | 6.10±1.16aγ | 3.92±2.14aγ | 15.40±3.20aγ | 100 |
| 9 | Compd3f | 2.22±0.28aγ | 7.86±0.76aγ | 4.21±1.66bγ | 22.40±4.45aγ | 80 |
| 10 | Compd3g | 2.41±0.12aγ | 9.66±1.02aγ | 3.98±2.26aγ | 20.50±3.60aγ | 80 |
| 11 | Compd3h | 2.32±0.34aγ | 8.26±0.82aγ | 4.43±2.0bγ | 21.33±4.82aγ | 80 |
| 12 | Compd3i | 2.36±0.16aγ | 8.84±0.68aγ | 4.82±2.86bγ | 19.34±2.86aγ | 80 |
| 13 | Compd3j | 2.10±0.76aγ | 7.64±0.922aγ | 4.64±3.23bγ | 22.56±3.94aγ | 80 |
Anticonvulsant Activity Against INH-induced Convulsions
Several 6-aryl-4,5-dihydropyridazinones were screened for their in-vivo ACA against isoniazid (INH, anti-TB drug) induced convulsion. Two series of 6-(4-Cl-Phenyl)-4-arylidene-4,5-dihydro pyridazin-3(2H)-one (3a-e), 6-(4-CH3-Phenyl)-4-arylidene-4,5-dihydro-pyridazin-3(2H)-one derivatives (3f-j), were evaluated as anticonvulsant against INH-induced convulsion method. The result indicated that all the compounds exhibited ACA. Compounds 3d and 3j showed maximum activity in 4-methyl-phenyl and chloro-phenyl compounds. Methyl derivatives were more active than chloro derivatives (Table 2). For ACAs PHT sodium (25mg/kg) and sodium vaproate (VPA) (50mg/kg) were used as reference drugs.
|
S. No. |
Groups |
Onset of convulsion (sec) |
No. of convulsion |
Recovery (%) |
|---|---|---|---|---|
| 1 | Compd 3a | 36.2±6.27 γa | 2.5±0.22 | 80 |
| 2 | Compd 3b | 47±4.50 γc | 2.8±0.25 | 80 |
| 3 | Compd 3c | 48.6±3.87γc | 2.5±0.24 | 60 |
| 4 | Compd 3d | 34.6±3.24γ | 2.4±0.36 | 100 |
| 5 | Compd 3e | 35.2±6.98γ | 2.6±0.35 | 100 |
| 6 | Compd 3f | 42.6±2.62 γc | 2.0±0.32 | 80 |
| 7 | Compd 3g | 46.8±2.06γc | 2.4±0.34 | 80 |
| 8 | Compd 3h | 48±1.82 γc | 2.4±0.26 | 80 |
| 9 | Compd 3i | 47.6±4.24 γc | 2.2±0.24 | 60 |
| 10 | Compd 3j | 48.2±4.18 γc | 2.6±0.18 | 100 |
| 11 | Sod. VPA | 130.2 ± 2.15 | 1.4±0.22 | 100 |
| 12 | PHT sod. | 0.00 | 0.00 | 100 |
| 13 | Control | 26.16±.094 | 3.2±0.20 | 00 |
Synthesized compounds have been evaluated for anticonvulsant activities by MES and INH induced convulsion methods. Results indicated that these compounds were shown good ACAs.
DISCUSSION
The nitrogen containing heterocyclic compounds is becoming more popular as an area of research because of their diverse biological activities. Pyridazine compounds have diverse biological activities. They are inexpensive and easily synthesized. Different substitutions on the pyridazine ring may cause noticeable differentiation in the biological activities. Due to these characteristics different substituents are being synthesized and evaluated for various biological activities in the search of improved therapeutic molecules. At present, much concentration has been focused on the pyridazine moiety attached with other heterocyclic ring, which has a valuable structure for molecular investigation and for the development of biological active molecules with diverse biological activities [38-43].
Possible Mechanisms
The discovery and development of a new chemical entity for the treatment of epilepsy rely on the use of experimental animal models. Currently there are two in vivo models that are commonly used by most AEDs discovery schemes. The models are MES and chemo convulsion induced seizure models. The MES and INH seizure models represent the two animal seizure models. To promote investigate the effects of the ACA in some other animal experiment models and estimation of neurotransmitters to consider about the exact possible mechanism of these compounds. PHT is inhibited sodium ion channel. There is some evidence that VPA enhances the action of GABA by a postsynaptic action. It also has effects on sodium channels, weaker than those of PHT [44].
Structural Feature of Pyridazinones as Anticonvulsant Activities
The anticonvulsant activities of pyridazinone compounds are known, there is a need for the advance of a molecule which have antiepileptic actions. Compounds active against MES-induced convulsions are working as ionic channel inhibitor (primarily Na+ channel inhibitor). Compounds active against INH-induced convulsions are acting as GABA antagonist. Results revealed that all compounds inhibited both ionic channel and GABA concentrations in the brain. Literature exposed that different pyridazinone analogs are active against epilepsy were exhibited significant anticonvulsant action [45, 46].
CONCLUSION
In this study, various substituted 3(2H)-pyridazinone compounds were synthesized, characterized and evaluated as anticonvulsant against various convulsion models.
- Substituted 3(2H)-pyridazinone compounds, 4-benzylidene-6-(4-substituted)-4,5-dihydro pyridazin-(2H)-one (3a-j) were synthesized.
- All the synthesized 3(2H)-pyridazinone compounds were characterized by spectral analysis, such as IR, NMR, mass spectra, and elemental analysis.
- All the synthesized 3(2H)-pyridazinone compounds did not exhibit any neurotoxicity up to 100 mg/kg dose levels.
- All the synthesized 3(2H)-pyridazinone compounds were evaluated against MES and INH induced convulsions at 50 mg/kg dose level. In MES activity, all compounds (3a-e) and (3f-j), compounds 3e and 3j were exhibited highest anticonvulsant activity. In isoniazid (INH) induced convulsion, all compounds (3a-j) exhibited appreciable activity. Compounds 3d and 3j showed maximum activity. Methyl derivatives were more active than chloro- derivatives. Methyl derivatives were more active than chloro and nitro derivatives. Phenytoin sodium (25mg/kg) and sodium vaproate (50mg/kg) were used as reference drugs.
- Result indicated that all these synthesized compounds were active as anticonvulsant these compounds were found active against MES, INH induced convulsion models.
- The MES method is sensitive to sodium (Na+) channel inhibitors (phenytoin), which compounds may inhibit voltage-gated ion channels (particularly Na+ channels). The INH (a GABA synthesis inhibitor) was sensitive to modulate γ-amino butyric acid (GABA), compounds may inhibit the GABA concentration in the brain.
The pyridazine moiety constitutes an important class of drug for new drugs research and developments. A variation in the substitution of the pyridazinone ring often causes a marked difference in the biological activities due to different characteristics of the substituent. The conclusion is based on the observation, regarding the synthesis of new pyridazinone compounds with the hope to obtain more potent drugs because easily functionalized different structural alterations were carried out in pyridazinone ring containing molecules. These structural modifications give outcome of some fruitful biological actions [47, 48].
Directions for Future Research
Many synthetic heterocyclic compounds have different biological activities. Pyridazine ring is an essential structural feature of various pharmacologically active compounds. Pyridazine also holds considerable interest in the synthesis of various organic intermediates as well as physiologically active agents. Pyridazinone compounds could be a suitable moiety to develop new lead antiepileptic agents with a minimum or without side effects as well as resistance. These findings encourage the synthesis of some pyridazinone derivatives and evaluate them for anticonvulsant activities. These pyridazinone derivatives are more efficient against seizures. The new pyridazine derivatives may be a promising agent as anticonvulsant in the future. Several synthetic strategies were reported for the synthesis of pyridazinone compounds. These structural alterations will result in some useful biological activities.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors are thankful to the Uttarakhand technical University, Dehradun, GRD (PG) IMT, Dehradun, India, Sophisticated Analytical Instrument Facilities, and Central Drug Research Institute, Lucknow, U.P, India for providing financial as well as technical support and facilities to carry out this work.
REFERENCES
| [1] | Asif M, Acharya M. Lakshmayya, Singh A. In silico physicochemical bioactivities and toxicities prediction of 3-chloro-6-arylpyridazines and 6-aryl-4,5-dihydropyridazine-3(2H)-thiones having anti-tubercular activity. RGUHS J Pharm Sci 2015; 5(2): 81-7. |
| [2] | Asif M. Bronchodilator and positive inotropic activity of pyridazine compound Zardaverine as a phosphodiesterase isozymes inhibitor. J Modern Med Chem 2015; 3: 31-4. |
| [3] | Asif M. A mini review on biological activities of pyridazinone derivatives as antiulcer, antisecretory, antihistamine and particularly against histamine H3R. Mini Rev Med Chem 2015; 14(13): 1093-103. |
| [4] | Asif M. The study of pyridazine compounds on prostanoids: Inhibitors of COX, cAMP Phosphodiesterase, and TXA2 Synthase. J Chem 2014; 703238 Available at: https://www.hindawi.com/journals/jchem/2014/703238/ |
| [5] | Asif M, Singh A.
Lakshmayya. Analgesic and anti-inflammatory activities of several 4-substituted-6-(3’-nitrophenyl)pyridazin-(2H)-3-one derivatives. Braz J Pharm Sci 2013; 49(4): 903-9. |
| [6] | Asif M. Antifeedant, herbicidal and molluscicidal activities of pyridazinone compounds. Mini Rev Org Chem 2013; 10(2): 113-22. |
| [7] | Asif M. Some recent approaches of biologically active substituted pyridazine and phthalazine drugs. Curr Med Chem 2012; 19(18): 2984-91. |
| [8] | Asif M, Singh A, Siddiqui AA. The effect of pyridazine compounds on the cardiovascular system. Med Chem Res 2012; 21: 3336-46. |
| [9] | Asif M, Anita S. Lakshmayya, Siddiqui AA, Husain A. Anti-inflammatory and antinociceptive activities of 6-phenyl (3’-imino-benzylidene)-4-benzylidene-2,3,5-trihydro-3-(2H)-pyridazin-3-one compounds. Acta Pharm Sci 2011; 53: 563-75. |
| [10] | Siddiqui AA, Islam M, Asif M, Asthana C. Synthesis and anticonvulsant activity of 6-(3’-nitrophenyl)-4-substitutedbenzylidene-2,3,5-trihydro pyridazinon-3-ones. J Ultra Fine Chem 2007; 3(2): 173-8. |
| [11] | Abdelrazek FM, Michael FA, Mohamed AE. Synthesis and molluscicidal activity of some 1,3,4-triaryl-5-chloropyrazole, pyrano[2,3-c]pyrazole, pyrazolylphthalazine and pyrano[2,3-d]thiazole derivatives. Arch Pharm (Weinheim) 2006; 339(6): 305-12. |
| [12] | Abouzid K, Abdel Hakeem M, Khalil O, Maklad Y. Pyridazinone derivatives: design, synthesis, and in vitro vasorelaxant activity. Bioorg Med Chem 2008; 16(1): 382-9. |
| [13] | Allerton CM, Andrews MD, Blagg J, et al. Design and synthesis of pyridazinone-based 5-HT(2C) agonists. Bioorg Med Chem Lett 2009; 19(19): 5791-5. |
| [14] | Cao S, Qian X, Song G, Chai B, Jiang Z. Synthesis and antifeedant activity of new oxadiazolyl 3(2H)-pyridazinones. J Agric Food Chem 2003; 51(1): 152-5. |
| [15] | Bialer M. New antiepileptic drugs that are second generation to existing antiepileptic drugs. Expert Opin Investig Drugs 2006; 15(6): 637-47. |
| [16] | Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs: a summary of the Seventh Eilat Conference (EILAT VII). Epilep Res 2004; 61: 1-48. |
| [17] | Chisholm D. Cost-effectiveness of first-line antiepileptic drug treatments in the developing world: a population-level analysis. Epilepsia 2005; 46(5): 751-9. |
| [18] | Hainzl D, Parada A, Soares-da-Silva P. Metabolism of two new antiepileptic drugs and their principal metabolites S(+)- and R(-)-10,11-dihydro-10-hydroxy carbamazepine. Epilepsy Res 2001; 44(2-3): 197-206. |
| [19] | Gerlach AC, Krajewski JL. Antiepileptic drug discovery and development: What have we learned and where are we going? Pharmaceuticals 2010; 3: 2884-99. |
| [20] | Banarjee PS, Sharma PK, Nema RK. Synthesis and anticonvulsant activity of pyridazinone derivatives. Inter J Chem Tech Res 2009; 1(3): 522-5. |
| [21] | Ghogare JG, Bhandari SV, Bothara KG, et al. Design, synthesis and pharmacological screening of potential anticonvulsant agents using hybrid approach. Eur J Med Chem 2010; 45(3): 857-63. |
| [22] | Wagh RV, Antre RV, Oswal RJ, Nimje HM. Anticonvulsant activity: An overview. J Pharm Res & Bio Sci Res 2011; 1(3): 142-7. |
| [23] | Oya U, Ayla B, Cenk A, Berna TM, Zafer G. Synthesis and evaluation of the anticonvulsant activities of some 5-(4-substitutedbenzylidene)-6-methyl-4,5-dihydropyridazine-3(2H)-ones. Fabad J Pharm Sci 2004; 29: 185-94. |
| [24] | Meenakshi D, Pravin S, Ashok B, Kapil J, Rahul D, Pramod S. Synthesis and evaluation of phenytoin derivatives as anticonvulsant agents. Turk J Chem 2009; 33: 1-7. |
| [25] | Asif M, Singh A. Lakshmayya, Husain A, Siddiqui AA. Anticonvulsant and antitubercular activities of 6-Phenyl/Biphenyl-4-yl-2-[2-(pyridin-2-ylamino)-ethyl]- and 6-(Biphenyl-4yl)-2-(2N-subtituted amin-1-yl)-ethyl derivatives of 4,5-dihyropyridazin-3(2H)-one. Lett Drug Des Discov 2013; 10(7): 651-60. |
| [26] | Siddiqui AA, Mishra R, Shaharyar M. Synthesis, characterization and antihypertensive activity of pyridazinone derivatives. Eur J Med Chem 2010; 45(6): 2283-90. |
| [27] | Asif M, Singh A. Lakshmayya. In-vivo anticonvulsant and in-vitro antimycobacterial activities of 6-aryl pyridazine-3(2H)-one derivatives. Am J of Pharmacol Sci 2014; 2(1): 1-6. |
| [28] | Asif M, Singh A. Lakshmayya. In-vivo anticonvulsant and In-vitro Antitubercular activity of methyl indole derivative of some 6-aryl-4,5-dihydropyridazin-3(2H)-ones their expected anticonvulsant mechanisms. Iran J Pharm Sci 2013; 9(1): 67-80. |
| [29] | Gul HI, Calis U, Vepsalainen J. Synthesis and evaluation of anticonvulsant activities of some bis Mannich bases and corresponding piperidinols. Arzneimittelforschung 2002; 52(12): 863-9. |
| [30] | Asif M, Singh D, Singh A. Analgesic activity of some 6-Phenyl-4-Substituted Benzylidene Tetrahydro Pyridazin-3(2H)-Ones. Global J Pharmacol 2011; 5(1): 18-22. |
| [31] | Asif M, Singh A. Synthesis and anti-tubercular activity of 6-(4-Chloro/Methyl-phenyl)-4-Arylidene-4,5-dihydropyridazin-3(2H)-one derivatives against Mycobacterium tuberculosis. Lett Drug Des Discov 2015; 12(6): 500-4. |
| [32] | Samanta KC, Asif M, Garg PV, Sharma P, Singh R. Synthesis of different substituted pyridazinone derivatives and their anticonvulsant activity. Eur J Chem 2011; 8(1): 245-51. |
| [33] | Asif M. Synthesis and analgesic activity of 6-(3’-nitrophenyl)-4-sustituted benzylidene-4,5 dihydropyridazin-3(2H)-one derivatives. Indonesian J Pharm 2013; 23(4): 254-8. |
| [34] | Asif M, Singh A. Lakshmayya. Anticonvulsant activity of 4-(substituted benzylidene)-6-(3-nitrophenyl)-4,5-dihydro pyridazin-3(2H)-ones against maximal electro shock induced seizure. Middle-East J Sci Res 2011; 9(4): 481-5. |
| [35] | Kulkarni SK. Handbook of Experimental Pharmacology. 3rd ed. New Delhi: Vallabh Publication 1999; pp. 131-4. |
| [36] | Unsal O, Balkan A, Aypak C, Terzolu B, Goren MZ. Synthesis and evaluation of the anticonvulsant activities of some 5-(4-substitutedbenzylidene)-6-methyl-4,5-dihydro pyridazine-3(2H)-ones. Fabad J Pharm Sci 2004; 29: 185-94. |
| [37] | Gobnitzer E, Krbavcic A, Wendelin W, Krbavcic M. Synthesis and structure investigations of potential sedative and anticonvulsant hydroxy- and acetoxy-N-(3-oxobutyl)-pyrido[2,3-d] pyridazinones. Monatsh Chem 2002; 133(9): 1177-85. |
| [38] | Asif M. General study of pyridazine compounds against cyclooxygenase enzyme and their relation with analgesic, anti-inflammatory and anti-arthritic activities. Chron Young Sci 2010; 1(3): 3-9. |
| [39] | Costas T, Besada P, Piras A, et al. New pyridazinone derivatives with vasorelaxant and platelet antiaggregatory activities. Bioorg Med Chem Lett 2010; 20(22): 6624-7. |
| [40] | Islam M, Siddiqui AA, Rajesh R. Synthesis, antitubercular, antifungal and antibacterial activities of 6-substituted phenyl-2-(3í-substituted phenyl pyridazin-6í-yl)-2,3,4,5-tetrahydropyridazin-3-one. Acta Pol Pharm 2008; 65(4): 441-7. |
| [41] | Rathish IG, Javed K, Bano S, Ahmad S, Alam MS, Pillai KK. Synthesis and blood glucose lowering effect of novel pyridazinone substituted benzenesulfonylurea derivatives. Eur J Med Chem 2009; 44(6): 2673-8. |
| [42] | Rakib EM, Abouricha S, Hannioui A, Benchat N, Ait M’Barek L, Zyad A. Synthesis and in vitro cytotoxicity studies of novel triazolo[4,3-b]pyridazinones. J Iran Chem Soc 2006; 3(3): 272-6. |
| [43] | Malinka W, Redzicka A, Lozach O. New derivatives of pyrrolo[3,4-d]pyridazinone and their anticancer effects. Farmaco 2004; 59(6): 457-62. |
| [44] | Luszczki JJ. Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions. Pharmacol Rep 2009; 61(2): 197-216. |
| [45] | Asif M. Anticonvulsant and comparative structure activity relationship of pyridazine derivatives with currently clinically used anticonvulsants. J Adv Sci Res 2010; 1(1): 35-45. |
| [46] | Sivakumar S, Surendra NP, James PS, Suthakar G. Anticonvulsant and sedative-hypnotic activities of N-Acetyl/Methyl Isatin derivatives. Sci Pharm 2008; 76: 621-36. |
| [47] | Asif M, Singh A. Exploring potential, synthetic methods and general chemistry of pyridazine and pyridazinone: A brief introduction. Inter J Chem Tech & Res 2010; 2(2): 1112-28. |
| [48] | Thaku AS, Verma P, Chandy A. A review on biological profile of pyridazinone containing drugs. Asian J Res chem 2010; 3(2): 265-71. |














