RESEARCH ARTICLE
Preparation and Evaluation of Multi-Particulate System (Pellets) of Prasugrel Hydrochloride
Navjot Kanwar, Rakesh Kumar, V.R. Sinha*
Article Information
Identifiers and Pagination:
Year: 2015Volume: 2
First Page: 74
Last Page: 80
Publisher Id: PHARMSCI-2-74
DOI: 10.2174/1874844901502010074
Article History:
Received Date: 26/12/2014Revision Received Date: 3/10/2015
Acceptance Date: 27/10/2015
Electronic publication date: 18/12/2015
Collection year: 2015
open-access license: This is an open access article licensed under the terms of the (https://creativecommons.org/licenses/by/4.0/legalcode), which permits unrestricted, noncommercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Multiparticulate systems (pellets) of prasugrel hydrochloride were prepared by extrusion spheronization method using MCC (micro crystalline cellulose). Optimum spheronization time and method of drying were selected as the process parameters for the preparation of final batches. Various pellet properties were evaluated like size & shape analysis, flow properties, bulk & tapped density, friability, moisture content, drug content, in vitro release rate and in vivo pharmacodynamic studies. All pellet batches showed a narrow particle size distribution, good sphericity and excellent flow properties. Drug content and moisture content of different pellet batches were found in specified limits. The release kinetics of drug loaded MCC pellets followed Peppas model with Fickian diffusion of prasugrel from the pellets. In vivo pharmacodynamic studies exhibited improved bleeding time in pellet group when compared with the marketed tablet formulation.
1. INTRODUCTION
Multiparticulate drug delivery systems (like pellets, granules, etc.) provide better gastrointestinal distribution and transportation resulting in minimizing the peak plasma fluctuations and side effects related to drug which are major advantages over single unit systems. Also, these delivery systems facilitate co-administration of incompatible drugs (using coated pellets) [1-3].
Pellets are oral dosage forms which are spherical beads with a mean particle size between 0.5 to 2 mm. Pellets are pharmaceutical formulations in which the active pharmaceutical ingredient is present as a number of small discrete units, each exhibiting some desired characteristics [4].
Pellets provide flexibility of delivery system, i.e. either be filled in capsules or compressed into tablets. Other advantages include lower irritative effects due to decreased local concentration and lower the risk effect due to dose dumping [5-8]. Pellets reduce the intra- and inter-subject variability in drug concentration by reducing the gastric emptying rate and transit time [9].
Extrusion-spheronisation is one of the most common and widely accepted methods for preparation of pellets in pharmaceutical technology [10, 11]. It is a multi-step process which produces pellets of good physical strength, uniform diameter and good porosity. The main advantage of pellets prepared by extrusion-spheronization method over other methods is the ability to incorporate high levels of active ingredient without producing excessively large particles [12, 13].
Prasugrel hydrochloride is a prodrug and a potent platelet inhibitor. It is an oral thienopyridine with rapid onset of action, consistent antiplatelet activity and prolonged duration of effect. It is used for reducing thrombotic cardiovascular events in patients with acute coronary syndromes managed with percutaneous coronary intervention [14].
In the present study, the effect of various process parameters (extrusion speed and spheronisation time) and drying methods (oven, vacuum oven and microwave oven) used in the preparation of prasugrel hydrochloride pellets (using extrusion-spheronisation method) were analyzed.
2. MATERIALS AND METHODS
2.1. Materials
Prasugrel hydrochloride was a generous gift from MSN Laboratories Limited, Bollaram, India. Avicel grade (MCC) was obtained from Ankit Pulps and Pvt. Ltd. All other chemicals and reagents used were of analytical grade.
2.2. Preparation of Pellets
Pellets were prepared using ethyl alcohol (absolute alcohol and with 5% water), propyl alcohol and distilled water as granulating liquid. Amount of granulating liquid (40 mL) used for preparation of pellets was taken as constant. Powder mass (100 gm) was moistened with 40 mL of the granulating fluid and extruded through the extruder with sieve no. 8# at 3 rpm and then spheronized in a spheronizer at 1000 rpm for 3 min. Extruder 25 and spheronizer 120 manufactured by Caleva process solution limited were used for pellet preparation. Effect of operational parameters, i.e. spheronization time and method of drying were studied on pellet properties. Optimum parameters were selected for the preparation of final batches of pellets. Extruder speed was kept constant. Wet pellets thus obtained were dried in the microwave for 2 min. Batch F and G differed in drug loading. Batch F contained 2% w/w drug whereas batch G contained 1% w/w drug.
The amount of granulating liquid used was kept constant for all the batches. 100 g powder mixture was taken and the dough was prepared using 40 mL water as granulating liquid. No other excipient was used for pellet formation. This wet mass was then extruded through the extruder at constant speed and subsequently spheronised on a spheroniser at a specific speed and time. Wet pellets thus obtained were dried with the help of microwave oven for 2 min.
2.3. Characterization of Pellets
2.3.1. Size Analysis
Size analysis of the pellet batches was carried out by Malvern Mastersizer 2000 (Malvern Instruments Ltd, Worcestershire, UK.). Pellets, which passed through 16# sieve, but retained on 25# sieve were taken for size analysis.
2.3.2. Shape Analysis
At least 20 pellets from each batch were randomly selected from the fraction obtained from size analysis. Pellets were mounted on a light microscope fitted with a camera and images of pellets were taken. Maximum and minimum radii of pellets were determined after measuring their respective diameters. Using these radii various shape factors were calculated as given below:
Roundness = Area/4π(maximum radius)2
Elongation = Maximum radius/Minimum radius
Pellips = 4 x Perimeter/2π(maximum radius)2
Rectang = Area/4(maximum radius x minimum radius)
2.3.3. Bulk and Tapped Density
Pellets were poured gently through a glass funnel into a 50 mL graduated cylinder until pellets just touched the 50 mL mark and the weight of cylinder required for filling the cylinder volume was determined. The cylinder was then tapped from the height of 2 cm until there was no more volume change. Bulk density was calculated as the quotient of the weight of pellets and volume of cylinder used. Tapped density was calculated as the quotient of the weight of the pellets and its volume after tapping.
2.3.4. Carr’s Index and Hausner Ratio
Using bulk and tapped densities Carr’s index (IC) and Hausner ratio (HR) was determined using formulae given below:
HR = Tapped density/Bulk density
IC = Tapped density-Bulk density/Tapped density
2.3.5. Flow Rate
The flow rate was calculated as the time taken for 10 g of pellets to flow through a funnel of 5 mm internal diameter. All the determinations were repeated in triplicate and the average flow rate with standard deviation was expressed in g/sec.
2.3.6. Angle of Repose
Angle of repose is a measure of flow properties of powder or pellets. The pellets were poured gently through a funnel, which was fixed at a position such that its lower tip was at the height exactly 2 cm above a hard surface. The pellets were poured until the upper tip of the pile surface touched the lower tip of the funnel. The tan-1 of (height of the pile/radius of its base) gave the angle of repose.
2.3.7. Friability
Accurately weighed (5 g) pellets were taken and placed in Roche’s friabilator and rotated for 200 revolutions at 25 rpm. Twelve steel balls (diameter 6.3 mm, weighing 1.028 gm each) were used to provide attrition. After friability, pellets were sieved through sieves. The weight loss (%F) after friability testing was calculated by the formula given below
% Friability = (Initial weight-Final weight/Initial weight) x 100
2.3.8. Moisture Content
The sample (5g) was placed in the infra-red moisture balance and heated using infra-red lamp. After complete removal of water present in the samples, the percentage moisture content was obtained from the instrument.
2.3.9. Drug Content
A known weight (500 mg) of the powdered pellets was dispersed in 100 mL of methanol. This was further diluted 10 times and filtered. The drug in the solution was analyzed using UV-visible spectrophotometer at λmax of 254 nm. All the determinations were done in triplicate. The drug has an appreciable solubility in methanol.
2.3.10.. Dissolution Studies
A sieve fraction of the pellets which pass through 16# but retained on 30# were used to study the drug release. 500 mg of the pellets having particle size 779.12-866.21 μm were used for dissolution studies. 500 mg pellets were equivalent to 10 mg and 5 mg of drug in batch F and G respectively.USP dissolution apparatus type 2 was used at a speed of 75 rpm. Aqueous solution containing 1% SLS was used as dissolution medium. Samples were taken at different time intervals and analyzed using UV spectrophotometry at λmax of 254 nm. The same volume of fresh aqueous solution containing 1% SLS was replaced in dissolution beaker. Dissolution studies were performed in triplicate and average drug release with standard deviation was calculated.
2.3.11.. In Vivo Pharmacodynamic Studies
Prasugrel hydrochloride, an anti-platelet drug was used to calculate the bleeding time in animals. The test was performed using male SD rats weighing 120-210 g. The animals were housed in accordance with the guidelines of institutional animal care and use committee (IACUC). All the animals were acclimatized to the laboratory conditions before use and feed on a standard normal pellet diet and water. Animals were divided into 3 groups, i.e. Group I was taken as a control, Group II was treated with pellets formulation and Group III was treated with marketed formulation.
Male SD rats were fasted overnight before the experiment. After one hour of the treatment with the test formulations (Pellets and marketed tablets), Ketamine (75mg/kg) was given i.p. to anesthetize the rats. The tail was transected 3mm from its tip with a razor blade and then immersed in a 15 mL clear conical tube containing normal saline pre-warmed to 37°C. Time for blood flow cessation (defined as no bleeding for 15 sec) was measured [15].
2.3.12.. Statistical Analysis
Simple analysis of variance (one-way ANOVA, GraphPad Prism 5) was used to determine statistically significant differences between the results and values with p<0.05 were considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. Preparation of Pellets
3.1.1. Granulating Agent
Non-aqueous vehicle (Alcohol): When absolute alcohol was used as granulating fluid, pellets formation failed that might be due to the lack of swelling of MCC (in the presence of ethyl alcohol). Similar results observed when isopropyl alcohol was used as granulating liquid. However, ethyl alcohol containing 5% water led to the formation of pellets, but on drying pellets crumbled due to lack of cohesion [16].
Aqueous vehicle (Water): MCC pellets were successfully formed using water as granulating liquid. However, pellets developed brown coloration when dried in oven at 40°C (Fig. 1). Hence, water is considered as the choice of granulationg fluid over ethyl alcohol and isopropyl alcohol in preparation of MCC pellets.
 |
Fig. (1). Drug loaded MCC pellets (Batch F) using water as granulating agent and dried in (a) oven at 40°C for 15 min (b) microwave for 3 min. |
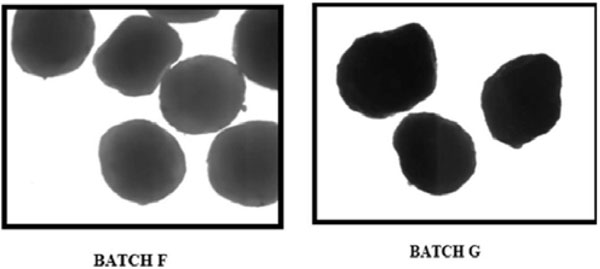 |
Fig. (2). Microscopic evaluation of pellet batches F and G (magnification 10X). |
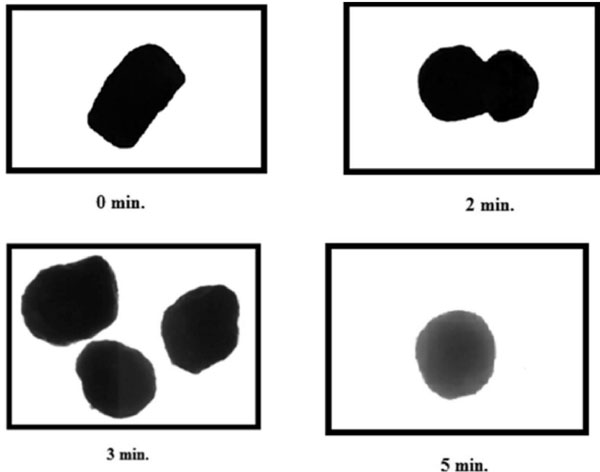 |
Fig. (3). Photomicrographs of extrudes [without spheronization (0 min), after 1 min (dumb-bell shaped), 3 min and 5 min of spheronization] (magnification 10X). |
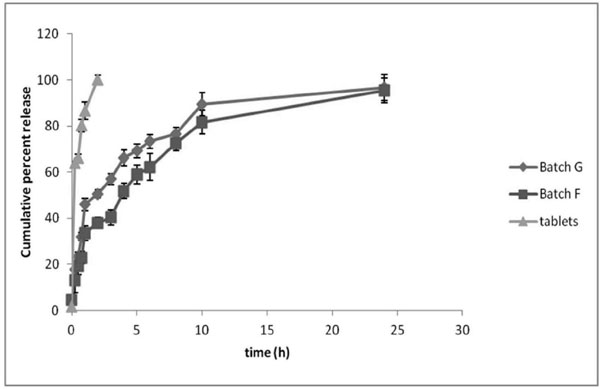 |
Fig. (4). In-vitro release of prasugrel HCl from pellets. |
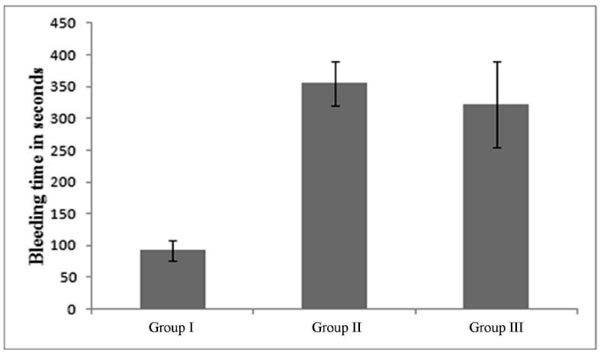 |
Fig. (5). Comparison of bleeding time in animal groups. |
Different granulating fluids and drying methods used for pellet preparation.
| S No. | Granulating fluid | Observation | Drying method | Inference |
|---|---|---|---|---|
| 1. | Absolute alcohol | Pellets didn’t form | - | Insufficient binding |
| 2. | Isopropyl alcohol | Pellets didn’t form | - | Insufficient binding |
| 3. | Ethyl-alcohol containing 5% water |
Pellets were formed but crumbled on handling |
- | Insufficient binding |
| 4. | Water | Pellets formed | Oven | Poor aesthetic appeal (Discoloration of pellets) |
| 5. | Water | Pellets formed | Microwave | Unchanged aesthetic properties |
Prasugrel release kinetics from selected batches.
| Kinetic model |
Zero order Dt = D0 + K0t |
First order ln Dt = ln D0 + K1t |
Higuchi Dt = D0 + KHt1/2 |
Korsemeyer-Peppas Dt/D∞ = KP tn |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Batch | R2 | K0 | R2 | K1 | R2 | KH | R2 | KP | n |
| F | 0.61 | 3.43 | 0.94 | 0.139 | 0.89 | 20.3 | 0.98 | 0.38 | 0.28 |
| G | 0.74 | 3.66 | 0.96 | 0.127 | 0.94 | 20.02 | 0.97 | 0.45 | 0.22 |
where Dt is the amount of drug released at time t, D0 is the initial amount of drug released, Dt/D∞ is fraction of drug released at time t, k0 is the zero-order release constant, k1 is the first-order release constant, kH is the Higuchi release constant, KP is the Peppas release constant, and n is the release exponent.
3.1.2. Drying Methods
It could be concluded that the discoloration (brown coloration) of pellets might have developed due to drying method (prolonged heating in the oven). Hence, different drying methods were used to prevent discoloration of pellets.
Vaccum oven: Drug loaded MCC pellets were prepared using water as granulating fluid and dried in vacuum oven at 5°C and 15 mm Hg. However, brown coloration of pellets was also observed under these conditions.
Microwave oven: No change in aesthetic properties of pellets were observed when dried in a microwave oven (Fig. 1). Unlike conventional oven, miro-oven utilizes micrwave radiations for drying and the duration of exposure was also lower which allowed complete drying of pellets without affecting any physio-chemical properties.
Based on the above observations, drug loaded MCC pellets using water as granulating fluid and microwave oven as drying method was selected as the optimized batch (Table 1).
3.2. Evaluation of Pellets
3.2.1. Size Analysis
Size distribution of pellets is an important characteristic as it affects the release kinetics of pellets [17]. Narrow particle size distribution is an indicator of uniformity in particle size. Polydispersity i.e. width of particle size distribution is measured by span. Span value less than 1.5 indicates narrow particle size distribution. The large span value indicates the variation in particle size, which results in segregation and non-uniform coating of pellets [18]. Arithmetic and geometric mean diameter are parameters for determining the size of pellets.
Particle size distribution curves were obtained from Malvern microsizer 2000 by dry method. Bell-shaped curves were obtained showing normal distribution, i.e. Gaussian curve. Mean diameter of pellets d(0.5) range from 779.12μm (batch F) to 866.21 μm (batch G) as shown while the Span value of different pellet batches was found to be 0.68 and 0.73 for batch F and G, respectively. Span value for all the pellet batches was found to be less than 1.5 which indicated narrow particle size distribution.
3.2.2. Shape Analysis
Roundness of pellet batches F and G was found as 0.92±0.05 and 0.92 ±0.04, respectively, which confirms the good sphericity of pellets as shown in Fig. (2). Roundness or sphericity enhances the uniformity of pellet shape that facilitates uniform/smooth coating of pellets. Higher value of Pellips and elongation indicates lower sphericity of pellets [19]. Elongation of pellet batches F and G was found as 1.09±0.06 and 1.09±0.04, respectively. Pellips and rectang values for pellet batches F and G were found to be 0.002±0.0003 & 0.002±0.0002, and 0.78±0.01 & 0.79±0.02, respectively.
Experiments were performed in the present studies to describe the mechanism of spheronization and the effect of spheronization time on shape of pellets after 2, 3 and 5 min. Initially, after extrusion the shape of extrudes was elongated which became dumb-bell shape after spheronizing the extrudes for 2 min. However, spherical pellets were formed after a spheronization period of 3 to 5 min as shown in Fig. (3). Hence, the sphericity of the pellets increased with spheronization time but no further changes in the roundness of pellets were observed after 5 min. Hence, the optimum spheronization time was selected as 5 min. The increase in roundness/sphericity with spheronization time could be explained by the fact that the extrudes were exposed to attrition forces for a longer period that resulted in improved sphericity. But after an optimum time period (5 min), pellets become rigid enough so that attrition forces have no further significant impact on the shape of pellets [20].
3.2.3. Bulk and Tapped Density
Tapped and bulk density for pellet batches were found as 0.57 & 0.69 g/cm3 (batch F) and 0.56 g/cm3 & 0.69 g/cm3 (batch G). Carr index and Hausner ratio for both the batches (F & G) were found as 1.30 and 1.01 % respectively. On comparing the values of Carr index and Hausner ratio given in USP 2011 with the experimental outcome, it was found that the flow properties of all batches were excellent.
3.2.4. Flow Properties of Pellet Batches
Pellets with high spheronization time showed good flow properties as compared to pellets with the low spheronization time that could be attributed to the improved sphericity of pellets with increasing spheronization time. The angle of repose for pellets batch F and G was 28.10±1.42° and 30.36±0.23° respectively. Similarly, the flow rate of pellet batches F and G was found as 2.82±0.24 and 2.64±0.09 g/sec, respectively. These values indicated an excellent flow of pellet batches.
3.2.5. Friability of Pellet Batches
Friability of pellets helps in determining the hardness and strength of pellets. MCC pellets produced by extrusion spheronization method have low friability. The friability of pellet batches F and G was found to be 1.80 % and 0.60 % respectively.
3.2.6. Moisture Content of Pellet Batches
The moisture content of prasugrel pellet batches was found close to 2%, which was within the prescribed limit. Moisture content is a parameter to evaluate the quality of the pellets. Moisture content below 1% indicates high friability of pellets (i.e. pellets break easily upon crushing) while higher moisture content (>3%) increases stickiness of pellets.
3.2.7. Drug Content of Pellet Batches
Drug content of pellets batch F and G was found between 97.28±0.10% and 98.53±1.92% respectively.
3.2.8. In Vitro Release Studies of Pellets
Batch F and G showed a burst release of 37.96±2.31 and 50.61±1.77% respectively in first two hours while sustained release was observed for the batch F (95.54±5.32%) and G (96.70±5.73%) after 24 hr (Fig. 4). No significant difference (p>0.05) was observed between the release profile of batches F and G. The release data of batch F and G was fitted to different kinetic models, viz. zero order, first order, Higuchi model and Korsmeyer-Peppas model. Among the kinetic models employed, Korsemeyer–Peppas showed a better fit as the R2 value was comparatively higher than the other kinetic models (Table 2). According to Peppas equation drug release from formulation occurs by diffusion of the drug from the formulation. The mechanism of diffusion deviates from the Fickian equation and follows a Non-Fickian or anomalous (irregular) behaviour in many cases, including the case of drug release from swellable polymeric systems. The values of release exponent, n observed using Peppas model was found as 0.28 and 0.22 for batch F and G, respectively suggesting Fickian diffusion of prasugrel from the pellets. In general the values of n ≤0.5, 0.5-1 and ≥1 are related to Fickian diffusion (case I transport), anomalous, and case II transport (zero order release), respectively [21-23]. Unlike optimized pellet batches, marketed formulation (tablet) exhibited immediate release (100.07±2.06 % in 2 hr).
As batch F has the lower burst release with sustained release profile, it was selected as the optimized batch for further studies.
3.2.9. In Vivo Pharmacodynamic Studies
Bleeding time provides a useful mean for estimation of platelet function. It helps in studying the in vivo effect of drugs which interfere with platelet aggregation [15]. In vivo pharmacodynamic efficacy of prepared formulation was evaluated and compared with marketed formulation (Prasita 10 mg-Tablet, Ranbaxy, Gurgaon, India). Bleeding test was performed to estimate the pharmacodynamic efficacy of different formulations. Animals were divided into three groups: Group I was taken as control Group, Group II was treated with pellets (batch F) suspended in 1%w/v methylcellulose and Group III was treated with marketed formulation. In Group I, after administrating 1% w/v methylcellulose suspension, a cut was made on the tail after 1h. The group I showed the bleeding time of 92.50±15.90 sec (Fig. 5) and was considered as the normal bleeding time in rats. Similarly, pellets suspended in 1%w/v methylcellulose and the marketed tablet were orally administered to the animals of group II and III respectively, and the bleeding time was noted as 355.00 sec (group II) and 321.75 sec (group III). It was found that pellets (Group II) significantly prolonged the bleeding time as compared to control group (Group I) and marketed formulation (Group III). The increased bleeding time in pellet formulation as compared to tablet might be due to the increased bioavailability (as a result of sustained drug release) of the drug resulted in improved therapeutic efficacy.
CONCLUSION
The multi-unit particulate system (pellets) of prasugrel hydrochloride was successfully developed and characterized. The extrusion-spheronization and the microwave oven used for fabrication and drying of pellets respectively, helped in the development of optimized pellet batches. The optimized formulation (Batch F) displayed narrow size distribution, good sphericity, low friability, higher drug content with a sustained release profile. Apart, the optimized batch exhibited improved therapeutic activity when compared with the marketed formulation. Further, incorporating the pellets in a hard gelatin capsule could mask the bad odour of the drug and hence can improve patient compliance.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors wish to thank MSN Laboratories Limited, Bollaram, India and Ankit Pulps Pvt. Ltd. India that kindly provided the samples of prasugrel hydrochloride and Avicel respectively.













