RESEARCH ARTICLE
Biocidal Activities of Substituted Benzothiazole of Copper Surfactants over Candida albicans & Trichoderma harziamunon on Muller Hinton Agar
Neha Mathur1, *, Nisha Jain2, A.K. Sharma3
Article Information
Identifiers and Pagination:
Year: 2018Volume: 5
First Page: 24
Last Page: 35
Publisher Id: PHARMSCI-5-24
DOI: 10.2174/1874844901805010024
Article History:
Received Date: 24/5/2018Revision Received Date: 1/9/2018
Acceptance Date: 12/9/2018
Electronic publication date: 31/10/2018
Collection year: 2018
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Heterocyclic complexes in the current era are aimed at evaluating as new products that possess wide biological, pharmacological, agricultural, medicinal, industrial applications and many more highly significant uses. Although they have been known from long ago for their great biocidal significance, still the researchers pay a great attention for profiling the pharmacological view of novel macrocycles like benzothiazoles. Increasing number of microbial infectious diseases and resistant pathogens create a demand and urgency to develop novel, potent, safe and improved variety of antimicrobial agents.
Objective:
This initiates a task for current chemistry to synthesize compounds that show promising activity as therapeutic agents with lower toxicity. It is very necessary to introduce new and biologically safe and active drugs eco-friendly in nature and effective as antimicrobial agents.
Methods:
Transition metal complexes share an important place in this regards. Therefore in this paper we report the synthesis and characterization of transition metal complex of N/S ligand by FT-IR, NMR, ESR, elemental analysis, conductometric and magnetic moment measurements. The synthesized metal complexes were successfully investigated for biological activities namely antifungal.
Results:
Based on the results we pronounced biocidal activities of the novel complexes. Concerned complexes contribute diverse applications by being more economical, harmless, non -toxic and eco-environmental friendly.
1. INTRODUCTION
The chemistry of nitrogen donor ligands is a very interesting field. Nitrogen containing ligands have a tendency of complexation with the transition metal soaps and they are recognized as pharmacophores which have received great attention in drug discovery and lead optimization [1]. A review of past researches reveals that among N- donor ligands, phenylthiourea and 2-amino benzothiazole are well known compounds bearing N-C=S linkage. This linkage enables the metal complexes to possess a range of biological applications namely tranquilisers, sedatives, neuroleptic, anti-histamines, anti-helminthes, anti-inflammatory, antipsychotic, anti-viral, anesthetics, anti-malarial, antibacterial, antifungal, diuretic, anticancerous, anticonvulsant, antibiotic etc. Some nitrogen and sulphur ligands are used in therapy, photo-sensitisers, indicators-dyes and heat stabilizers [2, 3]. The exact information of the nature and structure of these aromatic compounds is of great significance for explaining their characteristics under different conditions. The benzothiazole ring is present in various marine and terrestrial natural compounds which have biological significance. Heterocycles containing thiazole moiety are present in many natural products such as epothilone A, bleomycin,lyngbyabellin A and dolostatin [4]. Being a heterocyclic compound, benzothiazole finds use in research as a starting material for the synthesis of usually larger bioactive structures. Its aromaticity makes it relatively stable, although as heterocycle, it has reactive sites which allow functionalization [5]. A large number of therapeutic agents are synthesized with the help of benzothiazole nucleus [6]. Thiazole antifungal are known as potent inhibitors of the cytochrome, monooxygenase in the process of fungal biosynthesis of ergosterol, which is an important constitution of fungal cell membrane [7]. Metal co-ordination complexes have been widely studied for their antifungal, antibacterial and anticancer properties [8]. Many drugs possess pharmacological and toxicological properties when administered in the form of metallic complexes [9]. This stimulates our interest to extend the studies to their cumulative form as complexes because biological activity is often enhanced by complexation [10]. Copper in particular has attracted the researchers. Copper is an essential micronutrient required by all life forms [11]. The present research is an important effort to understand the characteristic nature and biocidal application of copper complexes of nitrogen donor ligands. The treatment with copper complexes produce remarkable pharmacological effects which are not observed when the parent ligands or inorganic forms of copper are used. The use of transition metal complexes as therapeutic compounds has become more and more pronounced. In the present study antifungal activity was performed for the synthesized complexes CP(BTA)A and CP(BTA)NA) on Muller Hinton Agar against Candida albicans, Trichodermaharzianum.
Abbreviation of complexes are as follows:
S3= CP[BTA] A Complex of copper palmitate with 2-amino-6-methoxy benzothiazole
S4 = CP[BTA]NA Complex of copper palmitate with 2-amino-6-nitro benzothiazole
2. EXPERIMENTAL
Copper soap and substituted benzothiazole ligand were prepared by earlier reported methods [12]. Their analytical and physical data of ligand, soap and complexes are summarized in Tables 1-4. The synthesized complexes are characterized by chromatography and spectroscopic techniques as follows:
| Compound | Molecular Weight | M.P. (°C) | Yield % | Colour | % Found (Calculated) | ||||
| C8H8ON2S | 180.24 | 134 | 98 | Sap green | C | H | O | N | S |
|
53.28 [53.33] |
4.4 [4.44] |
8.8 [8.88] |
15.5 [15.55] |
17.71 [17.77] |
|||||
| Compound | M. W. | M.P. (°C) | Colour | Yield | % Found (Calculated) | ||||
|---|---|---|---|---|---|---|---|---|---|
| - | - | - | - | C | H | O | N | S | |
| C7H5O2N3S | 195.11 | 202 | Black | 98 | 42.09 | 2.47 | 16.35 | 21.47 | 16.38 |
| - | - | - | - | - | [43.05] | [2.56] | [16.40] | [21.52] | [16.40] |
| Compound | M. W. | M.P. (°C) | Colour | Yield | % Found (Calculated) | ||||
|---|---|---|---|---|---|---|---|---|---|
| - | - | - | - | - | Cu | C | H | O | |
| C32H62O4Cu | 573.5 | 80 | Blue | 98 | 11.02 | 66.9 | 10.78 | 11.12 | 16.35 |
| - | - | - | - | - | [11.07] | [66.95] | [10.81] | [11.16] | [16.40] |
| Compound | M.F. | M.W. | M.P. (°C) | Yield | Colour | % Found (Calculated) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| - | - | - | - | - | - | Cu | C | H | O | N | S |
| CP[BTA]A | Cu2C80H140O10N4S2 | 1507.82 | 269 | 98 | Dark green | 7.97 | 64.52 | 9.35 | 10.66 | 3.38 | 4.11 |
| - | - | - | - | - | - | [8.42] | [63.72] | [9.28] | [10.60] | [3.71] | [4.24] |
| CP[BTA]NA | Cu2C78H134O12N6S2 | 1537.78 | 232 | 98 | Chatt black | 8.02 | 60.92 | 8.78 | 12.09 | 5.75 | 4.92 |
| - | - | - | - | - | - | [8.26] | [60.92] | [8.71] | [12.48] | [5.46] | [4.16] |
2.1. Chromatography
TLC is probably the most versatile technique for the analysis of complexes and their mixtures. TLC is quite efficient technique for separation and identification of synthesized soap, ligand and their complexes in sub microgram amounts using silica gel plates in various non-aqueous solvent systems. Rf of copper soap, ligands and the synthesized complexes in non-aqueous solvent systems has been determined. A thin layer chromatographic applicator was used for coating sorbent material on 20×3 cm glass plates to obtain absorbent layer of 0.25 mm thickness [13]. The coated plates were developed in glass jars of 24×6 cm. A glass sprayer was used to spray chromogenic reagent on the plate to detect the spot. Silica gel G acted as stationary phase whereas the following solvent systems were used as mobile phase.
- Acetone: Carbon tetrachloride
- Acetone: Petroleum ether
- Benzene: Acetone: Petroleum ether
Silica gel G and double distilled water are mixed in 1:3 (w/v) ratio with constant mechanical shaking until homogenous slurry was obtained to prepare the plates. Approx. 10μl of the test solution was applied on thin layer plates by using micropipette. The solvent ascent was fixed to 10-12 cm in all cases. When the development was completed, the plates are withdrawn from the jar and dried at room temperature. The spots were visible in day light.
2.2. Spectroscopy
The spectral measurements (IR and NMR) and elemental analysis were carried out at the “Regional Sophisticated Instrumentation Centre”, Central Drug Research Institute (RSIC, CDRI) Lucknow. The IR spectra of the complexes were obtained as KBr discs in the range 400-4000 cm–1 on Perkin Elmer spectrophotometer. 1H NMR spectra were recorded at CDRI, Lucknow using CDCl3 as reference. ESR spectra of the complexes were recorded at SAIF, IIT, Mumbai in the X-band region at microwave frequency 9.1 GHz and microwave power 5 mW under the magnetic field of strength 2000G at room temperature. Tetracynoethylene (TCNE) was used as the standard. Magnetic susceptibility measurements were conducted at S.P. University, Vallabh Vidhyanagar, Gujrat.
2.3. Biocidal
Step 1. Processing of the sample.
The unknown compound is re-suspended in DMSO in mass concentration (w/v)
Step 2. Antimicrobial Susceptibility Testing (Kirby-Bauer and Stokes' methods.) [14]
I. Hinton susceptibility test agar
a. SabouraudDextrose Agar (antifungal screening) is the only susceptibility test medium that has been validated by NCCLS. Mueller-Hinton agar should always be used for disk diffusion susceptibility testing, accordingly.
II. McFarland turbidity standard
A McFarland 0.5 standard was prepared and quality controlled prior to the beginning of susceptibility testing.
III. Preparation of inoculum
Preparation of inoculums is shown in Fig. (1) as schematic diagram.
 |
Fig. (1). Flow diagram for Antimicrobial study. |
IV. Inoculation procedure
This procedure was exactly same as the earlier reported methods [15].
V. Loading the plate with Positive, negative control and sample
The working supply of antibiotic (streptomycin, positive control) should be stored in the refrigerator (4°C). 50 µl of the antibiotic suspension was dispensed in the well labeled with C (control), (R, negative control), sample (S) to the plates as soon as possible, but no longer than 15 minutes after inoculation. Diffusion of the drug in the well begins immediately.
VI. Recording and interpreting results
After the Loading of C, S, R on the plate, the plate is inverted and incubated at 35°C for 16 to 18 hrs. After incubation, the diameter of the zones of complete inhibition were measured and recorded in millimeters.
Note:
- Di methyl sulfoxide (negative control) does not show any activity against test organism.
- Antifungal Sensitivity (Itraconozole 5 mg/well) serves as a positive control.
- Sample (unknown chemical compound) (5mg/ well (w/v))
3. RESULTS AND DISCUSSION
3.1. Chromatography
Results were reproducible with in ±0.02 Rf unit values for various substituted compounds using procedure for three solvent systems – the main part of the chromatographic studies was the attempt to obtain the standard conditions for their resolution and identification. The best shaped spots given by the solvents were considered to be suitable, and are A = Acetone = carbon tetrachloride, B = Acetone = Petroleum ether, C = Benzene = Acetone = Petroleum ether. In pure acetone spots run along with the solvent front hence could not be used [16].
Single spot was given by all compounds, uniform in shape in present investigation, so all of them are chromatographically pure. The results of copper palmitate, substituted benzothiazoles, phenylthiourea and complexes are given in Tables 5-7.
| S.No. | Molecular Formula | Solvent | Color of Spot | ||
|---|---|---|---|---|---|
| A | B | C | |||
| 1. | C32H62O4Cu | 54.3 | 52.1 | 51.7 | Blue |
B = Acetone = Petroleum ether
C = Benzene = Acetone = Petroleum ether
| S.No. | Molecular Formula | Solvent | Color of Spot | ||
|---|---|---|---|---|---|
| A | B | C | |||
| 1. | [BTA]A | 80.2 | 76.5 | 75.4 | Sap green |
| 2. | [BTA]NA | 78.5 | 75.4 | 73.8 | Black |
B = Acetone = Petroleum ether
C = Benzene = Acetone = Petroleum ether
| S.No. | Complex | Solvent | Color of Spot | ||
|---|---|---|---|---|---|
| A | B | C | |||
| 1. | CP[BTA]A | 88.0 | 85.7 | 83.9 | Dark green |
| 2. | CP[BTA]NA | 87.2 | 84.5 | 81.7 | Chatt black |
B = Acetone = Petroleum ether
C = Benzene = Acetone = Petroleum ether.
Acetone = Carbontetrachloride, Acetone, Petroleum ether and Benzene, Acetone = Petroleum ether and Benzene, Acetone = Petroleum ether were selected as best solvent systems for synthesized compounds. In benzene, with or without nitrobenzene, the spots were diffused and comet likes having tail against the flow of solvent. We have observed that Rf values for the series of compounds under investigation increased as the amount of benzene increased in the solvent system. It is observed from the tables that for all the ligands as well as complexes; nitro derivatives gave higher Rf values as compared to methoxy derivatives [17].
3.2. Spectroscopy
(1). IR Spectral studies
The relative intensities / peaks of IR spectra of synthesized complexes are shown in Tables 8-9
| Absorption Bands | CP[BTA]A (cm–1) | CP[BTA]NA (cm–1) |
|---|---|---|
| CH3 and CH2, C-H antisym. stretching | 2916 | 2919.0 |
| CH3 and CH2, C-H sym. stretching | 2845 | 2840.2 |
| N-H bonding | 1590 | 1595 |
| COO–, C–O antisym. stretching | 1515 | 1520 |
| COO–, C–O sym. stretching | 1468 | 1472 |
| CH2, C-H Bonding (δ) (twisting and wagging) |
1355 | 1390.2 |
| CH3, C-H rocking | 1120.8 | 1115.6 |
| CH2, C-H rocking | 728.4 | 722.8 |
| Cu-N stretching | 545 | 520 |
| Cu-O stretching | 502.2 | 490.2 |
| Absorption Bands | CP[BTA]A (cm–1) | CP[BTA]NA (cm–1) |
|---|---|---|
| NH2, N-H stretching | 3425 | 3460 |
| Ar-C-NO2 stretching | - | 1445 |
| N-C=S stretching | 1305 | 1320 |
| C=S stretching | 1175 | 1180 |
| Ar-C-OCH3asym. stretching | 1250 | - |
| Ar-C-OCH3 sym. stretching | 1020 | - |
| C-H, Deformation (“oop”) | 840 | 848.7 |
(A). Copper Palmitate
The IR spectrum of copper palmitate asymmetric stretching vibration of methyl and methylene group is observed at 2910 cm–1 whereas, the symmetric stretching vibration of the group occurred at lower frequency i.e. at 2840 cm–1. The absence of C=O band in the spectra of soap indicate that there is a resonance in the two C=O bonds of carboxylate group. The band in the region 485 cm–1 is due to copper to oxygen bond stretching vibration. This is called characteristic absorption of metal constituent of soap molecule.
(B). CP[BTA]A: Complex of copper palmitate with 2-amino 6-methoxy benzothiazole
The IR spectra of complex of copper palmitate with 2-amino 6-methoxy benzothiazole is shown in Fig. (2) and all IR peaks are summarized in Tables 8-9, it is confirmed from results that the characteristic band of metal constituent of soap molecule i.e. copper oxygen (Cu-O) stretching band has been distinguished at 502.2 cm–1. The strong band at 1604 cm–1 is the characteristic feature of the N-H bending vibration of the -NH2 group in the free ligand (2-amino 6-methoxy benzothiazole) but in this complex it is shifted to lower frequency at 1590 cm–1 indicating that the primary nitrogen is the coordinating site in the complex. This is further supported by the formation of new band at 545 cm–1, which is due to vM-N band.
 |
Fig. (2). IR Spectra IR Spectra of Complex: Copper (II) Palmitate with 2-Amino 6-Methoxy benzothiazole– CP[BTA]A. |
(C). CP[BTA]NA: Complex of Copper palmitate with 2-amino 6-nitro benzothiazole
The IR peaks of the complex of copper palmitate with 2-amino 6-methoxy benzothiazole are summarized in Tables 8-9, it is confirmed from results that the characteristic band of metal (copper) constituent of soap molecule i.e. (Cu-O) copper oxygen stretching band has been distinguished at 490 cm–1. A clear peak is observed at 1445 cm–1 corresponding to Ar-C-NO2 vibrations. An absorption band observed at 1320 cm–1 corresponds to N-C=S stretching.
(2). NMR Spectral studies
The relative signals of NMR spectra of synthesized complex are shown in Table 10.
| Peak / Signal | CP[BTA]A (δ) | CP[BTA]NA (δ) |
|---|---|---|
| –CH3-CH2-R | 0.87 | 0.91 |
| –CH2-CH2-R | 1.22 | 1.25 |
| –NH2 | 3.75-3.85 | 3.80-3.86 |
| Tautomeric –NH2 | 7.12 | 7.25 |
(A). CP[BTA]A: Complex of Copper palmitate with 2-amino-6-methoxy benzothiazole
Aliphatic -CH3 and -CH2 proton attached to -CH2-R group show signal at nearly δ 0.87 and δ 1.22 respectively. A broadened peak is observed at δ 3.75-3.85 corresponding to –NH2 proton. These 2 peaks indicate the co-ordination through the -NH, group of benzothiazole segment to the metal atom of the soap segment. The proton present on the nitrogen atom may undergo rapid, intermediate or slow exchange. The broadening of the observed peak is suggestive to be a slow exchange because the electrical quadripole moment of nitrogen nucleus induces a moderately efficient spin relaxation. A very weak signal is observed at δ 7.12 in the spectra, which may be due to aromatic protons on benzothiazole moiety.
(B). CP[BTA]NA: Complex of Copper palmitate with 2-amino-6-nitro benzothiazole
Aliphatic -CH3 and -CH2 protons attached to CH2-R group show signal at nearly δ 0.91 and δ 1.25 respectively. A broadened peak is observed at δ 3.80-3.86 corresponding to NH2 group. This peak indicates the co-ordination through the –NH2 group of benzothiazole segment to the metal atom of the soap segment. A very weak signal is observed at δ 7.25 in the spectra, which may be due to tautomerism and aromatic protons of benzothiazole moiety present in the complex [18].
(3). ESR Spectral studies
ESR spectrum is characterized by the position, intensity and shape of the component lines. The position of ESR is referred to as g-values and is directly determined by the energy levels. The variation in g-value is interpreted in terms of first and second order spin orbit interaction. The g-value for ESR signal is calculated by the formula [19]:
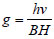 |
Where
v = Frequency of band in KHz
B = Bohr Magneton
H = Magnetic field
h = Plank's constant
On the basis of g|| and g| the values of gav and G were calculated using the following relations [20]
gav (g|| + 2 g┴)/3
G = g|| – 2/g┴ – 2
(A). CP[BTA]A: Complex of Copper palmitate with 2-amino-6-methoxy benzothiazole
The ESR parameters are recorded in Table 11 The values of g||, g┴ and gav are 2.30, 2.05, 2.103, 4.012 and greater than the value of g0 i.e. 2.00-2.77. This fact suggests that distortion from regular octahedron has taken place in the shape of the complex. From the observed value, it is clear that g||> g┴ > g0 for CP[BTA]A, which suggests that the complex exhibited in elongated octahedral geometry and the unpaired electron lies predominantly in the dx2-y2 orbital of Cu(II) giving 2B1g as the ground state. It is well known that g|| is 2.3 for copper nitrogen band.
| Complex | g|| | g┴ | gav | G |
|---|---|---|---|---|
| CP[BTA]A | 2.30 | 2.05 | 2.103 | 4.012 |
| CP[BTA]NA | 2.32 | 2.02 | 2.102 | 4.892 |
Since values are moderately sensitive function for indicating covalency, thus the value of g|| for complex of copper palmitate with 2-amino-6-methoxy benzothiazole is suggestive of covalent character of metal ligand. Since the value of G is greater than four for CP[BTA]A, it is suggestive of negligible exchange coupling interaction between the two copper centers in complexes.
(B). CP[BTA]NA: Complex of Copper palmitate with 2-amino-6-nitro benzothiazole
The value of g||, g┴ and gav are 2.32, 2.02 and 2.102 and 4.892 respectively. The value of g||, g┴ and gav are greater than the value of g i.e. 2.00-277. Also the trend g|| and g┴ for CP[BTA]NA indicates that the unpaired electron is most likely to be in dx2-y2 orbital of Cu (II) giving 2B1g as the ground state. This fact supports the statement that the complex possesses elongated octahedral geometry. Since g||value is a moderately sensitive function for indicating covalency, the value of g|| for benzothiazole complex is suggestive of covalent character of metal ligand band. Since the value of G is greater than four for complex, it is suggestive of negligible exchange coupling interaction between the two copper centers in the complex.
3.3. Magnetic Moment Studies
As it is reported earlier, the presence of copper ion leads to a magnetic moment of 1.73 BM. Various scientists have reported subnormal values for the magnetic moment of copper soap and fatty acid, inferring that the soap exists as a binuclear molecule. This has been attributed to the formation of δ-bonds arising from inter-molecular exchange demagnetization through lateral overlapping of 3dx2-y2 orbital on each copper ion.
The studies also reveal that these values of magnetic moments in the range 1.92-2.08 BM is due to the sub-dispersion unit of soap/complex which retains its binuclear configuration even in solution. It may be suggested that sub-dispersion units are bound together by weak polar forces resulting in the interaction between 3dxy and 3dxz orbital of two copper atoms [21].
The lower values of magnetic moments of copper ions in copper complex in benzene, as compared to those in solid state, reflect the terminals of binuclear molecules as [Cu2(RCOO)4L2] Where L represents the solvent molecule in solution, but is replaced by the ligand on complexation.
3.4. Conductivity Studies
The nature of the complexes is found to be non-electrolytic as the molar conductance values in benzene, lies in the range of 3.9-6.14 mhos cm2mol–1 [22].
3.5. Biocidal Studies
The study aims to evaluate the bioactivity of synthesized complexes against test organisms. Antimicrobial sensitivity was performed for synthesized complexes (Compound S3 and S4) on Muller Hinton Agar against Candida abicans, Trichodermaharzianumby Kirbyethod [23]
Results obtained using Itraconazole (5 mg (w/v)) as antifungal agent control (C) and for negative reference Ethanol/Methanol (R) are mentioned in Table 12 and Fig. 3)
| S. No | Microorganism | Sample | Zone of Inhibition (mm) | Mean Zone of Inhibition | Reference | Activity | |||
|---|---|---|---|---|---|---|---|---|---|
| Positive Control | Sample | ||||||||
| Antifungal Sensitivity | (Itraconozole mg/well) | ||||||||
| 1. | Candida albicans | [BTA]A | 19 | 8.5 | 9 | 9 | 8.83 | 10 | 0.868 |
| 2. | Trichoderma harzianum | 23.5 | 12 | 11.5 | 12 | 11.83 | 9 | 1.438 | |
| 3. | Candida albicans | [BTA]NA | 19 | 9 | 8 | 9 | 8.67 | 10 | 0.858 |
| 4. | Trichoderma harzianum | 23.5 | 11 | 10 | 10.5 | 10.5 | 9 | 1.178 | |
Sample I: Sample (5 mg/ well (w/v))
Diameter of the zone of inhibition is given.
Diameter of the well was 8mm.
Dimethyl Sulphoxide (negative control)
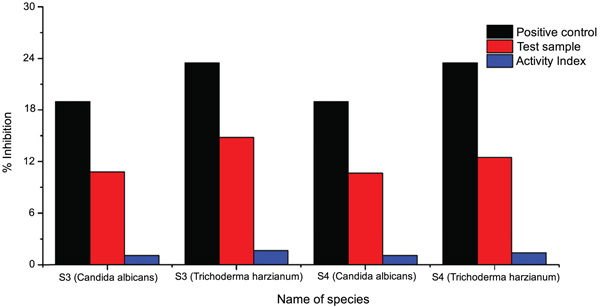 |
Fig. (3). Plots presenting the comparative study of complex sensitivity against different fungi under study. |
Activity index = zone of inhibition of sample[S]/zone of inhibition of reference [R] And the activity can be found out by [24]:
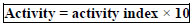 |
If the Zone of Inhibition is
< 13 = it means that the extract is inactive,
13 – 18 = it means that the extract is bioactive,
> 18 = it means that the extract is highly active.
Table 12 data reveals that complexes show higher activity than ligands suggesting that complexes are more powerful anti- fungal agents and benzothiazole and other N, S, O etc. containing compounds are able to enhance the performance of copper soaps. The enhanced activity of newly synthesized complexes as compared to those of the ligand can possibly be explained on the basis of chelate formation, presence of donor atoms, basicity as well as the structural compatibility with molecular nature of the toxic moiety [25].
The results of ANOVA for the antifungal activities for all complexes are shown in Table 13. The predicted R2 are in reasonable agreement and closer to 1.0. This confirms that the experimental data are well satisfactory. The descriptive static results of all complexes also confirm satisfactory results in triplet.
| - | Anova: Single Factor | - | - | - | - | - | - | - |
|---|---|---|---|---|---|---|---|---|
| - | SUMMARY | - | - | - | - | - | - | - |
| Complex | Groups | Count | Sum | Average | Variance | Std Deviation | Coff. Variance | Std Error |
| S3 | Candida albicans | 3 | 96 | 32 | 1.00 | 1.00 | 0.0313 | 0.018 |
| - | Trichodermaharzianum | 3 | 109 | 36 | 0.33 | 0.58 | 0.0159 | 0.009 |
| S4 | Candida albicans | 3 | 30 | 10 | 0.08 | 0.29 | 0.0284 | 0.016 |
| - | ANOVA | - | - | - | - | - | - | - |
| - | Source of Variation | SS | df | MS | F | P-value | F crit | - |
| - | Between Groups | 1180.16 | 2 | 590.083 | 1249.588 | 1.37385E-08 | 5.1433 | - |
| Within Groups | 2.833 | 6 | 0.472 | - | - | - | - | |
| Total | 1183 | 8 | - | - | - | - | - |
CONCLUSION
The antifungal activity of the synthesized complexes has been evaluated by the Muller Hinton Agar method. The results are expressed in millimeter. The two antimicrobial disks (Itraconozole for anti-fungal) were taken as standards and the sample disks were compared with it. A scrutiny of results reveals that both pure soaps and ligands show less inhibition whereas on complexation the inhibition enhanced and also complexes show higher activities than pure ligands suggesting that complexes are more powerful agents. Hence, we can conclude that Benzothiazoles and other N and S containing compounds are able to enhance the performance of copper soaps.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The authors express their sincere thanks to the Principal, Head of the Department of Chemistry, Pt. N.K.S. Govt. P.G. College, Dausa for providing laboratory facilities. Heartfelt thanks to Therachem laboratories, Jaipur for NMR spectral data, IIT Mumbai for FT-IR and ESR spectral data.














