RESEARCH ARTICLE
Improvement in Dissolution of Bosentan Monohydrate by Solid Dispersions Using Spray Drying Technique
Pankaj V. Dangre*, Vikesh B. Sormare, Mangesh D. Godbole
Article Information
Identifiers and Pagination:
Year: 2017Volume: 4
First Page: 23
Last Page: 31
Publisher Id: PHARMSCI-4-23
DOI: 10.2174/1874844901704010023
Article History:
Received Date: 10/01/2017Revision Received Date: 20/02/2017
Acceptance Date: 02/03/2017
Electronic publication date: 28/04/2017
Collection year: 2017
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Bosentan monohydrate (BM), a dual endothelin receptor antagonist, is indicated for the treatment of patients with pulmonary arterial hypertension (PAH). It is poorly soluble in water, and having absolute bioavailability of 50%.
Objective:
The aim of the present work is to develop and evaluate the solid dispersions (SD) of a poorly water soluble drug bosentan monohydrate (BM).
Method:
Solid dispersions (SDs) systems of BM were prepared with Hydroxy propyle β-cyclodextrin (HPβ-CD) and Polyethylene glycol (PEG-4000) polymers using a spray drying technique.
Result:
The significant rise in a saturation solubility 174.23±1.36 mg/mL; and drug dissolution 95.11±1.22%; was observed with optimized formulation (SD 6). The solid state characterization of optimized formulation (SD 6) by SEM, DSC, and XRPD revealed the absence of crystalline nature of BM in solid dispersion. High dissolution rate of solid dispersion (SD 6) compared with pure drug indicated the increase in dissolution characteristics.
Conclusion:
In conclusion, our studies illustrated that spray drying technique could be useful large scale producing method to prepare the solid dispersion of bosentan with HP β-CD, which can improve the solubility as well as stability of the formulation.
1. INTRODUCTION
Oral route is always favored and widely accepted for the administration of drug and it is firstly investigated in the development of new dosage form. With the recent advent of high throughput screening and combinatorial chemistry methods, the number of new chemical entities (NCE) that possess pharmacological activity has risen sharply and thus candidates for pharmaceutical development. However, it has been estimated that the majority of all NCE entering in pharmaceutical development programs possess insufficient aqueous solubility to allow complete gastrointestinal absorption at a magnitude sufficient to ensure therapeutic efficacy [1, 2].
A number of alternative technologies are being developed to overcome the drawbacks associated with poor aqueous solubility, for example, including use of surfactants [3], micronization [4], inclusion complexation [5] and solid dispersion [6]. Among these solid dispersion has gained importance as a promising approach, as the other methods suffer from limitation like size reduction by micronization, results in the formation of surface charge on particles which restrict their flow and exhibit poor flow properties [7]. Furthermore, drug solubilization from solid dispersion system is mainly due to particle size reduction, increased surface area, reduction in crystallinity and increased wettability by a surrounding hydrophilic carrier that improves the dissolution rate [8]. Among the solid dispersion techniques, spray drying offers numerous advantages over the other methods like; no drug and carrier decomposition (mostly occurred during fusion process in melting method) [9], no solvent limitation (as happened in case with solvent evaporation where the single organic solvent has solubility difference for drug and carrier employed for preparing solid dispersions) [10] and it is most suitable for large scale-up of products [11].
Bosentan monohydrate (BM), a dual endothelin receptor antagonist, is indicated for the treatment of patients with pulmonary arterial hypertension (PAH) [12]. It is poorly soluble in water, and having absolute bioavailability of 50% [13]. Therefore, improvement in solubility and/or dissolution rate of bosentan may be achieved through the formation of solid dispersion. Literature reported on the enhancement of solubility of bosentan monohydrate by using mixed hydrotropy approach [14].
In the present study, spray drying technique was utilized to prepare a solid dispersion of bosentan with hydroxy propyl β-cyclodextrin (HPβ-CD) and polyethylene glycol 4000 (PEG 4000). Spray drying technique successfully employed for the improvement in dissolution of indomethacin [15], tolbutamide [16], carbamazepine [17], ketoprofen [18], and albendazol [19]. Solid dispersion in the form of spray dried powder was characterized by drug content, saturation solubility, dissolution rate, Scanning Electron Microscopy (SEM), Thermal analysis, Radiograph powder diffraction (XRPD) and Infrared spectroscopy (FT-IR).
2. MATERIALS AND METHODS
Bosentan monohydrate was provided by Cipla Pvt. Ltd. (Mumbai, India). Hydroxy propyl β- cyclodextrin (HPβ-CD) and polyethylene glycol (PEG 4000) was purchased from Loba Chem Ltd. (Mumbai, India). All chemicals and reagents used were of analytical grade.
3. PREPARATION OF SOLID DISPERSION
Bosentan was dissolved in methanol (10% w/v) along with each of the HPβ-CD and PEG 4000 in different ratios (1:1, 1:3, 1:5) of drug carrier. Spray drying technique was employed to prepare solid dispersions since it allows rapid solvent evaporation leading to fast transformation of drug: carrier solution to solid drug: carrier particles. Furthermore, spray drying technology has wider application in commercial pharmaceutical industry because it is simple, rapid and economical. The solutions were mixed well to obtain a clear solution. The clear solutions were spray-dried using a spray dryer (Labultima, Mumbai, India) under fallowing set of parameters i.e. Inlet temperature 80oC, outlet temperature 55oC, feed pump rate 8 mL/min, aspiration rate 45 m bar, atomization air pressure 2.5 kg/cm2. The resulting solid dispersions were subsequently desiccated under vacuum for 24 h to remove residual solvents. The dried mass then triturated and passed through sieve No. 80 to get uniformity in the size of the particles and were stored in tightly closed container until further use.
4. EVALUATION OF PREPARED SOLID DISPERSION
4.1. Drug Content Determination
The percentage of drug content in solid dispersions was estimated by dissolving the solid dispersions equivalent to 10 mg of bosentan in 100 mL of methyl alcohol. Each of these solutions ware further diluted with phosphate buffer (pH 6.8) and absorbance were measured at 273 nm.
4.2. Saturation Solubility Studies
To the glass vials containing 5 mL of phosphate buffer (pH 6.8) excess quantity of pure drug and each solid dispersions were added separately. These vials were shaken at 20 rpm for 24 h at temperature 37±0.5oC using orbital shaking cum incubator (Remi). The samples then filter through Whatman filter paper (No-41), appropriate dilutions were made with phosphate buffer (pH 6.8) and analyzed spectrophotometrically by measuring absorbance at 273 nm.
4.3. Drug Dissolution Studies
Dissolution studies of Bosentan and its solid dispersions were performed using a USP XXII type II dissolution test apparatus (Electrolab-TDT 08L). The samples equivalent to 32 mg of bosentan were placed in a dissolution vessels containing 900 mL of phosphate buffer (pH 6.8) maintained at 37±0.5oC and stirred at 100 rpm [20]. The aliquots of 5 mL were removed at predetermined intervals of time and replaced immediately with the fresh dissolution medium. The samples were filtered and analyzed spectrophotometrically by measuring absorbance at 273 nm.
4.4. Fourier Transforms Infrared Spectroscopy Studies (FTIR)
The FT-IR spectra of Bosentan, polymers and solid dispersions were obtained using FT-IR spectrometer having DRS attachment (84000s Shimadzu Corporation, Japan). About 2-3 mg of samples was mixed with dry KBr and the spectra scanned over wave number range of 4000- 400cm-1.
4.5. Differential Scanning Calorimetric Analysis (DSC)
Thermal behavior of pure drug, polymer and solid dispersion was studied by DSC (Mettle Star SW 10). The each sample was placed in aluminum crucible and heated at a rate of 10oC/min in the range of 45oC to 250oC. Inert atmosphere was provided by purging nitrogen gas at flow rate of 50 mL/min.
4.6. X-Ray Powder Diffractometry (XRPD)
X-ray diffraction pattern of selected solid dispersion was compared with that of pure drug. This was done by measuring the 2θ in the range of 4-40o with the reproducibility of ±0.001 on diffractometer (Brucker axs D8 Advance, Germany). The XRPD pattern was recorded automatically with the rate meter with the time constant of 2×102 pulse/second and with the scanning speed 2θ/min.
4.7. Scanning Electron Microscopy (SEM)
SEM analysis of selected solid dispersion was carried out by scanning electron microscope (JSM 6360A Jeol Ltd, Tokyo, Japan). The sample was mounted on a double adhesive tape stuck to an aluminum stub coated with the platinum. The sample was scanned and photomicrograph was taken at an acceleration voltage of 10 kv.
4.8. Stability Studies
The stability study was carried out on selected formulation (SD 6). The selected formulation sealed in aluminum packaging coated inside with polyethylene and various replicates were kept in humidity chamber (Bio-Technics, Ltd, India) maintained at 40 ± 2oC/ 75±5% RH for 3 months to assess its stability. After 45 and 90 days formulation was withdrawn and analyzed for physical appearance, drug content (%) and cumulative drug release (%).
5. RESULT AND DISCUSSION
The process conditions for spray drying were optimized accordance with the practical yield of dispersions.
5.1. Drug Content Determination
The percent yield of solid dispersion of bosentan with HPβ-CD and PEG 4000 was 67-70%, and 59-62% respectively. The spray dried dispersion was in the form of free flowing, hygroscopic, solid fine powder having 95.93 to 99.58% drug content as presented in Table 1, showing uniform distribution of drug in solid dispersion and good flow and compressibility characteristics.
| Sr. No | Batch code | Drug: Carrier |
Drug content (%) |
Saturation solubility (mg/mL) |
Cumulative drug release (%) after 60 min |
|---|---|---|---|---|---|
| 1 | Pure drug | -- | -- | 55.54±1.02 | 37.70±2.22 |
| 2 | SD1 | 1:1 (PEG 4000) | 95.77±1.48 | 88.72±1.63 | 73.28±1.21 |
| 3 | SD2 | 1:3 (PEG 4000) | 96.16±1.11 | 104.33±1.52 | 78.59±1.23 |
| 4 | SD3 | 1:5 (PEG 4000) | 99.23±0.86 | 123.63±1.73 | 92.25±1.35 |
| 5 | SD4 | 1:1 (HPβ-CD) | 95.39±0.78 | 110.24±1.99 | 82.75±1.45 |
| 6 | SD5 | 1:3 (HPβ-CD) | 95.43±0.43 | 138.16±1.69 | 90.25±1.54 |
| 7 | SD6 | 1:5 (HPβ-CD) | 99.58±1.18 | 174.23±1.36 | 95.11±1.22 |
5.2. Saturation Solubility Studies
Fig. (1) shows significant improvement in saturation solubility of solid dispersion over pure drug. The increase in solubility 55.5 to 174% was observed when the drug was formulated into solid dispersion using carrier HPβ-CD in 1:5 ratio. The presence of carrier i.e. HPβ-CD and PEG 4000 in sample solution shows no interference in absorbance of drug.
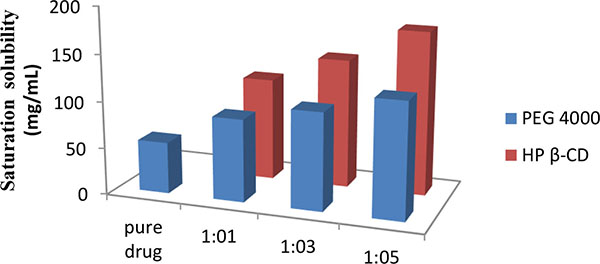 |
Fig. (1). Saturation solubility of solid dispersions. |
5.3. Drug Dissolution Studies
An increased saturation solubility of drug leads to improvement in dissolution of drug as per the Noyes and Whitney equation (Figs. 2 and 3). This could be attributed to the decreased crystallinity of bosentan as revealed by DSC and XRPD studies, and reduction in particle size. The reduction in particle size during the spray drying, increases the surface area of particles and thus promoting higher dissolution rate. HPβ-CD and PEG 4000 improve the wettability of drug particles and it acted on the diffusion layer surrounding the drug particles involving rapid inclusion of the solvent molecules thereby increasing the dissolution rate.
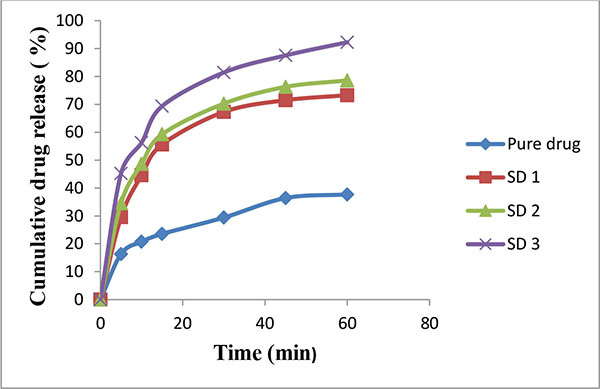 |
Fig. (2). Release rate profile of bosentan from solid dispersions prepared with PEG 4000 (Mean ± SD; n=3). |
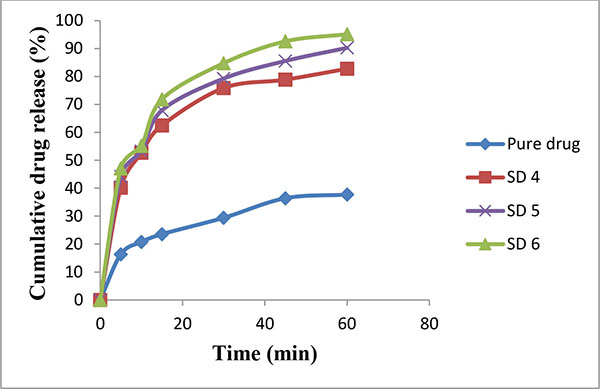 |
Fig. (3). Release rate profile of bosentan from solid dispersions prepared with HP β-CD (Mean ± SD; n=3). |
5.4. Fourier Transforms Infrared Spectroscopy Studies (FTIR)
The FT-IR spectrum (Fig. 4) of Bosentan showed characteristics peaks at 2960.73 cm-1 (CH3- stretching), 3493.09 cm-1 (OH- stretching), 1080.14 cm-1 (C-O stretching in ether) and 1384.89 cm-1 (S=O stretching). The FT-IR spectra of solid dispersion showed slight shift in lower wave number of stretching peaks 2927 cm-1 (CH3- stretching), 3383 cm-1 (OH- stretching) with no difference in overall spectrum. This observation reveals possibility of intermolecular hydrogen bonding in the solid dispersion.
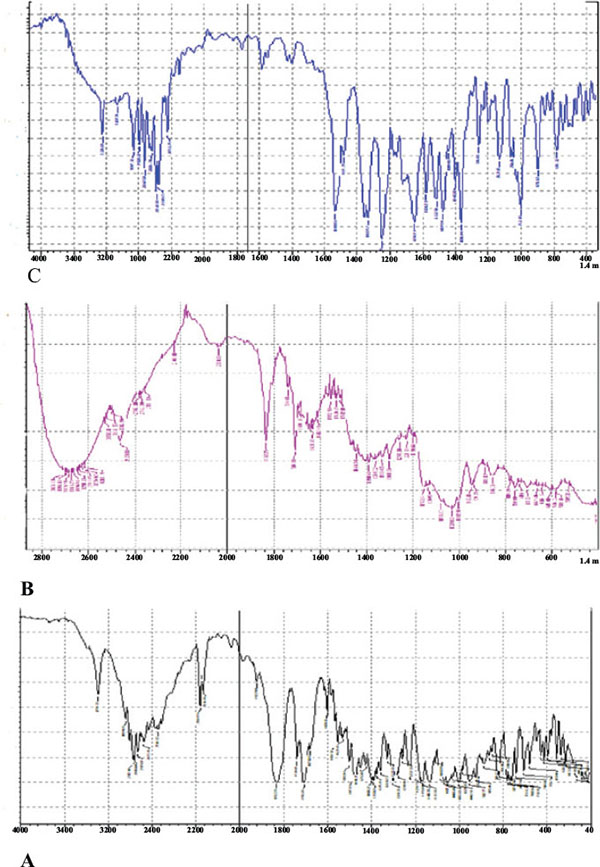 |
Fig. (4). Fourier-transform infrared (FTIR) spectroscopy spectra of bosentan (A), HP β-CD (B) and solid dispersion SD 6 (C). |
5.5. Differential Scanning Calorimetric Analysis (DSC)
The DSC curve of bosentan and its solid dispersion with HPβ-CD is shown in Fig. (5). The thermal curve of bosentan showed a sharp endothermic peak at 126.08oC corresponding to the melting point of drug. The sharp melting point of the drug indicates its crystalline characteristic. However, the DSC thermogram of solid dispersion (SD 6) showed a reduced and diffused endothermic peak. The diffused DSC pattern of solid dispersion (SD 6) revealed the shift in the endothermic peak and reduced intensity indicating the change in the crystalline nature of the drug. This transformation of the drug from crystalline nature to amorphous resulted in marked improvement in dissolution rate as the latter possess the high internal energy and considered as a state of high disorder.
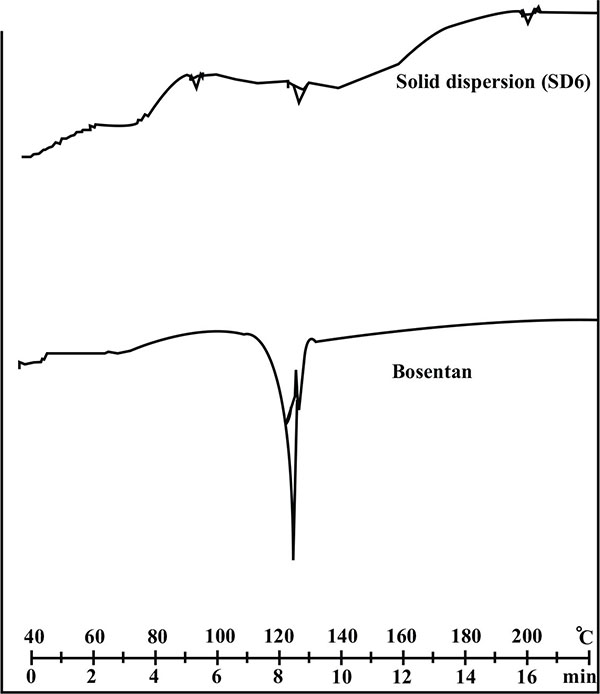 |
Fig. (5). Differential scanning calorimetry thermogram of pure drug and its solid dispersion with HPβ-CD (SD 6). |
5.6. X-Ray Powder Diffractometry (XRPD)
The XRPD pattern of drug, HPβ-CD and its solid dispersion is shown in Fig. (6). The XRPD pattern of drug was characterized by the presence of sharp peaks at 21.8o and 27.8o indicating crystalline nature of drug. The sharpness of peaks as well as number of sharp peaks existing with plain drug was found to be significantly reduced in solid dispersion (SD 6) suggesting loss of crystalline nature of the drug. This transformation clearly indicated that the drug exists in molecularly dissolved state in solid dispersion which further governs the effective solubilization of the drug.
5.7. Scanning Electron Microscopy (SEM)
The scanning electron microscopy (SEM) photographs HPβ-CD and solid dispersion is shown in Fig. (7). HPβ-CD is look like cup shape molecule with interior cavity. The interior of the cup is relatively a polar and creates hydrophobic microenvironment. HPβ-CD therefore has exterior hydrophilic environment and interior hydrophobic cavity responsible for improving the aqueous solubility of poorly soluble drug.
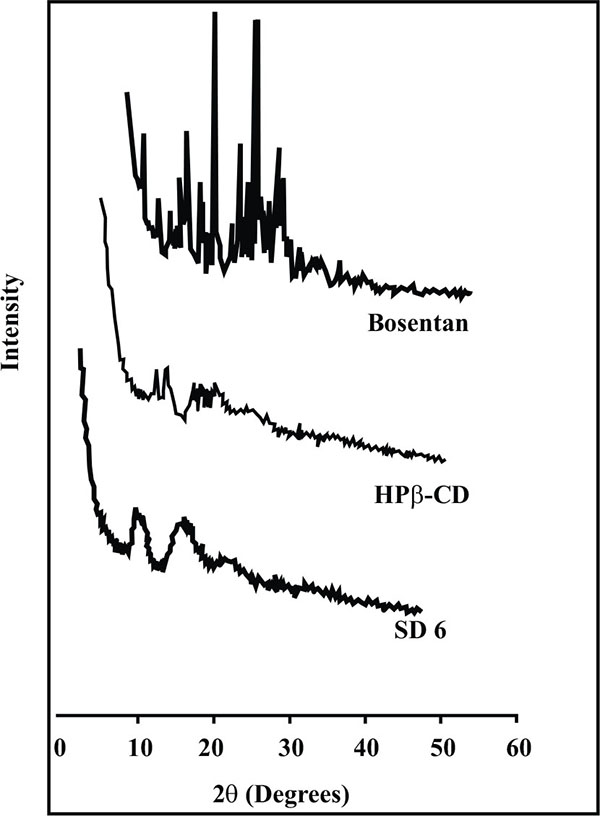 |
Fig. (6). X-ray powder diffraction spectra of Bosentan, HP β-CD and solid dispersion SD 6. |
| Initial | 45 days | 90 days | |
|---|---|---|---|
| Physical appearance | White color, Free flowing powder |
No change | No change |
|
Drug Content (%) |
99.58±1.18 | 98.18±1.12 | 97.86±1.32 |
|
Cumulative drug release (%) After 60 min. |
95.11±1.05 | 94.23±0.69 | 93.85±1.11 |
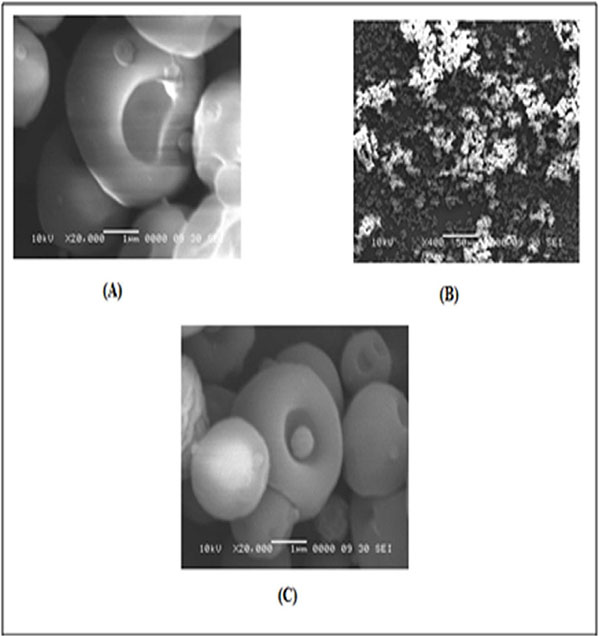 |
Fig. (7). Scanning electron microscopy photograph of HP β-CD (A), Inclusion complex of bosentan and HP β-CD in 1:5 ratio (B) and (C). |
The SEM images of solid dispersion showed inclusion of drug within their cavities forming inclusion complex. The incorporation of drug molecule inside HPβ-CD inclusion complex as a “guest” has been the basis for the improvement of solubility of drug.
5.8. Stability Studies
The stability studied was performed on selected solid dispersion formulation (SD 6). The formulation was stored at accelerated testing conditions for 3 months to assess its stability. The samples were withdrawn intermediately between 45 and 90 days of stability period and tested for physical appearance, drug content and percent cumulative drug release. On storage, stability of selected formulation (SD 6) showed no significant change as shown in Table 2. It is concluded that selected formulation was found to be stable over a period of three months.
CONCLUSION
The present investigation highlighted the simple and convenient method for preparation of solid dispersion of BM. The study proves that the solid dispersion of BM prepared using HPβ-CD and PEG 4000 has improved the dissolution rate of the drug significantly. However, better results were obtained with HPβ-CD over the PEG 4000 and therefore considered for further evaluation. Physical studies showed the absence of chemical interactions between the drug and polymer. The nature and the concentration of the hydrophilic polymers have a critical role in the enhancement of the solubility. Solid dispersion (SD 6) was found to be superior in dissolution enhancement. This indicated that an increase in mass fraction of the polymer significantly improve dissolution rate. Based on these results, it can be concluded that solid oral dosage form of bosentan with HPβ-CD could be formulated with a high dissolution rate, faster onset of action and can improved the bioavailability.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We are sincerely thankful to the secretary of Kamla Nehru College of Pharmacy, Butibori, Nagpur, India for providing the instrumental facility for carrying out the research work. We are also thankful to the Cipla Pvt. Ltd. Mumbai, India for providing the gift sample of Bosentan monohydrate.














