RESEARCH ARTICLE
Design, Synthesis and Evaluation of Substituted 3-(1, 4-Diazepanyl)-Methyl-Phenyl-Sulphonamides as Potent 5-HT6 Antagonists in Cognitive Disorders
V.S. Velingkar1, *, A.K. Chindhe2, Mrunal Sanaye2, Madhumangiri Gatane3
Article Information
Identifiers and Pagination:
Year: 2016Volume: 3
First Page: 138
Last Page: 148
Publisher Id: PHARMSCI-3-138
DOI: 10.2174/1874844901603010138
Article History:
Received Date: 23/12/2015Revision Received Date: 9/05/2016
Acceptance Date: 18/05/2016
Electronic publication date: 30/06/2016
Collection year: 2016
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Background:
3-(1, 4-diazepanyl)-methyl-phenyl-sulphonamides were prepared by reacting 3-nitrobenzaldehyde with substituted 1, 4-diazepanes which were reduced to obtain intermediate amines. Reaction of these amines with substituted sulfonyl chlorides using TEA in DCM followed by KOH in methanol afforded compounds which under HCl salt formation with IPA.HCl gave title compounds with good yields.
Objective:
To synthesize and evaluate 3-(1, 4-diazepanyl)-methyl-phenyl-sulphonamides as 5-HT6 Antagonists in Cognitive Disorders.
Method:
Melting points were determined in open capillary tube and are found uncorrected. The completion of organic reactions and purity of the compounds were checked by TLC on pre-coated Silica gel aluminum plates using a mixture of chloroform and methanol (8:2, v/v) as an eluent. UV light or iodine vapour was used for visualization. Infrared (IR) spectra were recorded (in KBr) on a Fourier-transform IR, model IR Affinity-1 (SHIMADZU), and the values are expressed in cm-1. The 1H NMR spectra were obtained on multinuclear FT NMR Spectrometer, model Advance-II (Bruker), (400 MHz) using CDCl3 as solvent, Tetramethylsilane (TMS) as an internal standard. The chemical shift values are expressed as ppm (parts per million) units, downfield from TMS.
Results:
Experimental results of synthesized derivatives were shown comparable activity to the earlier reported derivatives in literature on 5-HT6 antagonist therapeutic area.
Conclusion:
It can be concluded that test compounds have an ability to prevent loss of memory.
INTRODUCTION
5-Hydroxytryptamine 6 (5-HT6R) or serotonin receptor, the family member of G-protein coupled receptors (GPCRs) [1, 2] is present in various regions of the brain and is associated with learning and memory. Blockade of their function increases acetylcholine and glutamate mediated neurotransmission and enhances the cognitive process, which amply demonstrates the therapeutic usefulness of this receptor for CNS mediated disorders such as schizophrenia, Alzheimer’s disease, obesity and eating disorders [3-14].
Worldwide research efforts from several groups, have discovered several classes of serotonin 5-HT6 receptor ligands [15-18] with good affinity and selectivity. Lead molecules like SB-742457 for cognition, PRX-07034 for obesity and LY-483518 (SGS-518) for schizophrenia are in clinical studies and positive clinical study data has been reported [19-23].
MATERIALS AND METHODS
Designing of 3-(1, 4-Diazepanyl)-Methyl-Phenyl-Sulphonamides Using PHASE
Many aryl sulphonamides as 5-HT6 antagonist reported better affinity towards the receptor as per literature [24]. The understanding of common structural features of these aryl sulphonamides responsible for affinity was helpful for designing of novel entities.
A set of reported variously substituted analogues as 5-HT6 antagonist were selected for pharmacophore generation and atom-based 3D-QSAR analysis by using PHASE drug design software (Schrodinger, Inc). The study was carried out on Redhat Linux platform with processor of 2 GHz and memory 512 RAM. PHASE supports various ligand-based drug design approaches like pharmacophore perception, structure alignment, 3D-QSAR and database searching [25-27]. In silico receptor binding affinity (Ki) was predicted by 3D-QSAR model which was generated for the present dataset [25]. The PLS statistics for 3D-QSAR is shown in Table 1.
Statistical values for 3D-QSAR model generated by PLS.
| Training set | Test set |
|---|---|
| m = 6 | - |
| n = 33 | nT = 13 |
| R2 = 0.98 | Q2 = 0.67 |
| SD = 0.14 | RMSE = 0.38 |
| F = 281.50 | Pearson-R = 0.83 |
| P = 2.28e-22 | - |
A novel 3D database was prepared with various novel substitutions on aryl sulphonamide scaffold by maintaining the required pharmacophoric features. The novel 3D database analogues overlaid onto the best pharmacophore hypothesis AAPR. The novel 3-(1, 4-diazepanyl)-methyl-phenyl-sulphonamide analogues were exhibited good alignment with the derived pharmacophore model. In silico binding affinities (Ki) in nM with the receptor were predicted which are shown in Table 2.
5-HT6R novel ligands in silico binding affinities (Ki) nM and fitness scoresa.
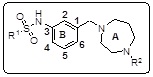
| Ligands | R1 | R2 | Fitness | Ki (nM) | Ligands | R1 | R2 | Fitness | Ki (nM) |
|---|---|---|---|---|---|---|---|---|---|
| 10a | Ph | H | 2.38 | 18.85 | 10g | i-Pr | H | 2.02 | 08.71 |
| 10b | 4-F Ph | H | 2.41 | 20.34 | 10h | Me | H | 2.09 | 17.41 |
| 10c | 4-Cl Ph | H | 2.41 | 14.46 | 10i | 4-Br Ph | H | 2.27 | 14.89 |
| 10d | 4-Me Ph | H | 2.41 | 10.00 | 10j | 4-F Ph | CH3 | 2.10 | 24.73 |
| 10e | 4-OCH3 Ph | H | 2.04 | 08.06 | 10k | 4-Cl Ph | CH3 | 2.09 | 19.95 |
| 10f | Et | H | 2.33 | 07.11 | 10l | 4-Me Ph | CH3 | 2.10 | 17.65 |
It was hypothesized that a positively charged basic amine functionality, like the terminal nitrogen of cyclic diamine, is essential for binding to the receptor and the nitrogen attached directly to the aromatic ring presumably help to impart the appropriate orientation for binding to 5-HT6R. It was postulated that a basic amine function, like the terminal nitrogen of 1, 4-diazepane which is shown in Table 2, would be essential for binding to the receptor and the flexible methylene spacer which is attached directly to the aromatic ring presumably would help to impart the appropriate orientation for binding to 5-HT6 receptor.
ADMET In Silico Screening Using QikProp
Various pharmaceutically relevant ADMET properties of novel 3-(1, 4-diazepanyl)-methyl-phenyl-sulphonamide analogues were predicted using QikProp-Schrodinger programme. The descriptor values were verified with the standard values which were reported in the literature are shown in Table 3. It was found that all the novel analogues showed significant values of the properties studied. All the compounds obey Lipinski’s rule of 5 violations, Jorgensen’s rule of three violations, good oral absorption and lipophilicity.
In silico ADMET predictions of best novel hit “Compound 10f”b.
| Compound “10f” | |||||
|---|---|---|---|---|---|
| #stars | 0 | Accpt HB | 8 | # metab | 3 |
| #rotor | 5 | QP polrz | 35.167 | QPlog Khsa | -0.18 |
| CNS | 1 | QPlogPoct | 19.375 | Human Oral Abs. | 2 |
| dipole | 6.351 | QPlogPw | 13.342 | % Human Oral Abs | 62.393 |
| SASA | 562.431 | QPlogPo/w | 0.977 | PSA | 69.289 |
| FOSA | 184.717 | QPlogS | -0.645 | Rule Of Five | 0 |
| FISA | 119.045 | QPP Caco | 45.793 | Rule Of Three | 0 |
| Donor HB | 2 | QPPMDCK | 21.872 | ||
| WPSA | 0.967 | QPlog BB | 0.057 | ||
Experimental
All LR grade chemicals were used in the synthetic experiment. Melting points were determined in open capillary tube and were found uncorrected. The completion of reaction and purity of the compounds were checked by TLC on pre-coated silica gel aluminium plates using a mixture of chloroform and methanol (8:2, v/v) as an eluent. UV light or iodine vapours were used for visualization. Infrared (IR) spectra were recorded (in KBr) on a Fourier-transform IR, model IR Affinity-1 (SHIMADZU) and the values were expressed in cm-1. The 1H NMR spectras were obtained on multinuclear FT NMR Spectrometer, model Advance-II (Bruker) (400 MHz) using CDCl3 as solvent. Tetramethylsilane (TMS) was used as an internal standard. The chemical shift values were expressed as ppm (parts per million) units, downfield from TMS. The Multiplicity of the NMR signals were designated as singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m).
Synthesis of 3-(1, 4-Diazepanyl)-Methyl-Phenyl-Sulphonamides
Synthesis of the proposed compounds was achieved as shown in Scheme 1. In general, 3-(1, 4-diazepanyl)-methyl-phenyl-sulphonamides (10a-10l) were prepared by reacting 3-nitrobenzaldehyde (1) with substituted 1, 4-diazepane (2, 5). Compounds (3, 6) were reduced with Iron powder-ammonium chloride or Iron powder-ammonium formate to obtain intermediate amines (4, 7). Reaction of these amines with appropriately substituted sulfonyl chlorides using TEA in DCM which was followed by KOH in methanol conditions afforded compounds (8, 9). In situ deprotection and HCl salt formation of compounds (8, 9) with IPA.HCl were given compounds (10a-10l) with good yields.
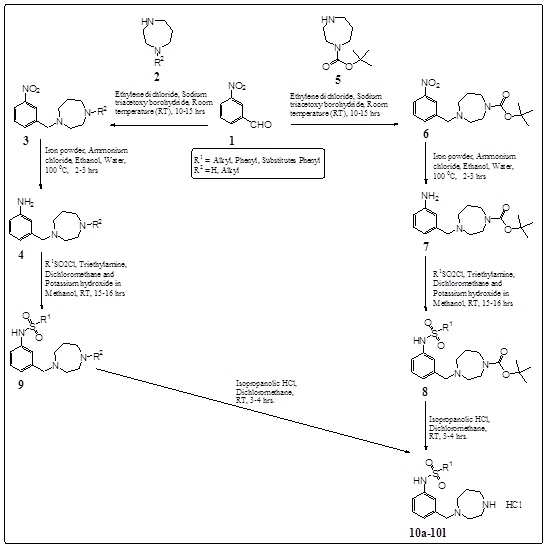 |
Scheme (1). Synthesis of 3-(1, 4-diazepanyl)-methyl-phenyl-sulphonamide derivatives. |
The compounds were evaluated for their binding affinity (Ki) nM on 5-HT6R through ligand based pharmacophore model development in silico screening using AAPR.10 developed pharmacophore model by PHASE-Schrodinger software. The Ki values and fitness scores of 3-(1, 4-diazepanyl)-methyl-phenyl-sulphonamides which were obtained from in silico screening of 5-HT6R are shown in Table 2. During ADMET properties prediction by QikProp-Schrodinger software, it was found that the entire novel designed derivatives were shown significant values of the ADMET properties studied. All the compounds were obeyed Lipinski’s rule of 5 violations, Jorgensen’s rule of 3 violations, good oral absorption and lipophilicity. ADMET predictions of best novel hit “Compound 10f” is given in Table 3.
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl) benzenesulfonamide hydrochloride (10a)
Molecular Formula: C18H23N3O2S; Molecular Weight: 345.46; Yield: 0.033 g. IR (KBr) v: 3344, 2981, 1593, 1481, 1338, 1087, 879, 690, 582 cm-1.
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl)-4-fluoro benzenesulfonamide hydrochloride (10b)
Molecular Formula: C18H22FN3O2S; Molecular Weight: 363.45; Yield: 0.016 g. IR (KBr) v: 3383, 2960, 2665, 1591, 1151, 1089, 837, 696, 663 cm-1; MS (70 eV) m/z %: 364 (M+H) +.
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl)-4-chloro benzenesulfonamide hydrochloride (10c)
Molecular Formula: C18H22ClN3O2S; Molecular Weight: 379.90; Yield: 0.011 g. IR (KBr) v: 3398, 3294, 2985, 1600, 1346, 1157, 1091, 624, 543 cm-1.
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl)-4-methyl benzenesulfonamide hydrochloride (10d)
Molecular Formula: C19H25N3O2S; Molecular Weight: 359.49; Yield: 0.012 g. 1H NMR (DMSO-d6, 400 Mz) δ: 2.26-2.39 (s, 3H), 3.23-3.42 (m, 11H), 3.49 (bs, 2H), 7.06-7.08 (d, 1H), 7.29-7.35 (m, 5H), 7.65-7.68 (m, 2H), 10.43 (s, 1H); IR (KBr) v: 3298, 3224, 2958, 1597, 1477, 1334, 1157, 1091, 894, 740 cm-1; MS (70 eV) m/z %: 360.1 (M+H) +
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl)-4-methoxy benzenesulfonamide hydrochloride (10e)
Molecular Formula: C19H25N3O3S; Molecular Weight: 375.49; Yield: 0.052 g. 1H NMR (DMSO-d6, 400 Mz) δ: 1.58-2.86 (m, 11H), 3.58 (bs, 2H), 4.20 (bs, 3H), 6.83-7.58 (m, 8H), 10.28 (s, 1H); IR (KBr) v: 3387, 2949, 2657, 2486, 1593, 1257, 1151, 1091, 802, 696, 665 cm-1; MS (70 eV) m/z %: 376.3 (M+H) +.
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl) ethane sulfonamide hydrochloride (10f)
Molecular Formula: C14H23N3O2S; Molecular Weight: 297.42; Yield: 0.011 g. IR (KBr) v: 3396, 2960, 2654, 1593, 1454, 1328, 1139, 696 cm-1; MS (70 eV) m/z %: 298.1 (M+H) +.
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl) propane-2-sulfonamide hydrochloride (10g)
Molecular Formula: C15H25N3O2S; Molecular Weight: 311.44; Yield: 0.012 g. IR (KBr) v: 3398, 3066, 2972, 2474, 1593, 1415, 1134, 694, 794, 696 cm-1.
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl) methane sulfonamide hydrochloride (10h)
Molecular Formula: C13H21N3O2S; Molecular Weight: 283.39; Yield: 0.011 g. IR (KBr) v: 3394, 2962, 2659, 2360, 1593, 1327, 1311, 1145, 974, 773, 698 cm-1.
N-(3-((1, 4-diazepan-1-yl) methyl) phenyl)-4-bromo benzenesulfonamide hydrochloride (10i)
Molecular Formula: C18H22BrN3O2S; Molecular Weight: 424.36; Yield: 0.023 g. IR (KBr) v: 3468, 3294, 2958, 1639, 1469, 1342, 1157, 894, 756 cm-1.
4-fluoro-N-(3-((4-methyl-1, 4-diazepan-1-yl) methyl) phenyl) benzenesulfonamide hydrochloride (10j)
Molecular Formula: C19H24FN3O2S; Molecular Weight: 377.48; Yield: 0.005 g. IR (KBr) v: 3360, 2916, 2848, 1681, 1602, 1157, 1093, 1008, 800, 617 cm-1.
4-chloro-N-(3-((4-methyl-1, 4-diazepan-1-yl) methyl) phenyl) benzenesulfonamide hydrochloride (10k)
Molecular Formula: C19H24ClN3O2S; Molecular Weight: 393.93; Yield: 0.010 g. IR (KBr) v: 2924, 2854, 1732, 1456, 1259, 1161, 1082, 1012, 800, 761, 611 cm-1.
4-methyl-N-(3-((4-methyl-1, 4-diazepan-1-yl) methyl) phenyl) benzenesulfonamide hydrochloride (10l)
Molecular Formula: C20H27N3O2S; Molecular Weight: 373.51; Yield: 0.012 g. 1H NMR (DMSO-d6, 400 Mz) δ: 2.48 (m, 6H), 2.87 (m, 10H), 3.47 (m, 2H), 7.08-8.13 (m, 8H), 10.30 (m, 1H); IR (KBr) v: 3319, 2943, 2866, 2831, 1454, 1022, 752, 665 cm-1; MS (70 eV) m/z %: 374.2 (M+H)+.
Animals
Albino Wistar mice weighing 20-30 gm body weight of male and female sex were procured from registered breeders Bharat Serum and Vaccines Ltd.Thane. The animals were maintained in hygienic conditions in our animal house in groups of 5-6 in clean plastic cages containing husk bedding. The animals were fed standard pellet food (Amrut brand pelleted standard feed manufactured by Nav Maharashtra Chakan Oils Ltd. Pune, Maharashtra) and water ad libitum. Constituents of feed pellet used: Crude proteins: 21.99%, Crude oils: 04.06%, Crude fibers: 03.22%, Ash: 07.22%, Sand silica: 01.32%. The animal house conditions maintained were: Temperature (22±1)0C, Relative humidity (65±10) % and 10 hr (light) and 14 hr (dark) cycle. The animals were allowed to acclimatize to our animal house conditions for 8-10 days period prior to the experiments. The institution’s animal house was registered with Govt. of India with registration No. 25/1999/CPCSEA and was conformed to the Indian National Science Academy guidelines for the use and care of experimental animal research. All the experimental protocols involving animal studies were placed before the Institutional Animal Ethics Committee. The committee was granted approval after carefully reviewing the research project.
Preliminary In Vivo Toxicological Evaluation [28]
2.0 gm test compound 10f was dissolved in 10 ml distilled water to prepare test solution. The test solution was administered to 6 Albino mice (3 females + 3 males) weighing in the range of 20-25 gm, at a dose of 2000 mg/kg. The mice were critically observed for clinical signs, gross behavioral changes and mortality if any, following the administration of the test formulation at different time intervals like 30 min, 1 hr, 2 hrs, 4 hrs, 24 hrs, 48 hrs and 72 hrs up to a period of 14 days. The study was carried out according to OECD guidelines No. 423.
In acute toxicity study, oral administration of representative test compound 10f at the dose up to 2000 mg/kg did not reveal any signs of toxicity, behavioural changes or mortality in male and female mice so those were found to be safe.
Elevated Plus Maze (EPM) In-Vivo Animal Model for Cognition in Alzheimer’s Disease [28, 29]
Albino Wistar mice were divided into 5 groups of 6 animals each viz:
- Group I: Control group (untreated)
- Group II: Toxicant group (Scopolamine 0.4 mg/kg, i.p.)
- Group III: Standard treatment group (Piracetam 200 mg/kg, p.o. + Scopolamine)
- Group IV: Test compound 10f treatment group (10 mg/kg, p.o. + Scopolamine)
- Group V: Test compound 10f treatment group (30 mg/kg, p.o. + Scopolamine)
The elevated plus maze animal model was consisted of two open arms (25 cm×5 cm) and two covered arms (25 cm×5 cm×14 cm) extended from a central platform (5 cm×5 cm) and the maze was elevated to a height of 25 cm from the floor. The standard and test samples were administered orally to the respective groups for 8 consecutive days, whereas toxicant scopolamine was administered 90 min after the administration of the last dose on 8th day. The animals were exposed to the training session after 45 min of scopolamine administration. In training session each mouse was placed at the end of an open arm, facing away from central platform and time taken by the animal to move from open arm into one of the covered arms with all its four legs which was in terms of Transfer Latency (TL) for each animal. The mouse was allowed to explore the maze for another 2 min and then returned to its home cage. Retention of this learned-task was examined after 24 hr in terms of TL i.e. on the 9th day and immediately animals in all groups were sacrificed using ether. The whole brain was removed aseptically and brain tissue homogenate was prepared in 0.1 M phosphate buffer solution (pH 7.4) which was further utilized for estimation of various biochemical parameters like acetyl cholinesterase (AChE).
It was used to evaluate learning and memory in mice. In groups treated with test compound 10f (10 mg/kg and 30 mg/kg) significant decrease in transfer latency was observed as compared to toxicant group which are shown in Table 4.
Vehicle Vs Toxicant (EPM): F=2.031, p=0.2277 by One tailed ANOVA, p<0.0001 shown non significant result
Vehicle Vs Toxicant (PAP): F=4.242, p=0.0694 by One tailed ANOVA, p<0.0001 shown non significant result
Every group except vehicle control was treated with scopolamine
Passive Avoidance Paradigm In Vivo Animal Modelfor Cognition in Alzheimer’s Disease [29, 30]
Albino Wistar mice were divided into 6 groups of 6 animals each viz:
- Group I: Control group (untreated)
- Group II: Toxicant group (Scopolamine 0.4 mg/kg, i.p.)
- Group III: Standard treatment group (Piracetam 200 mg/kg, p.o. + Scopolamine)
- Group IV: Test compound 10f treatment group (10 mg/kg, p.o. + Scopolamine)
- Group V: Test compound 10f treatment group (30 mg/kg, p.o. + Scopolamine)
Effect of Test compounds on Transfer latency in Elevated plus maze and Step down latency in Passive avoidance paradigm models in mice.
| Groups | EPM (TL in seconds) n=6 | PAP (SDL in seconds) n=6 |
|---|---|---|
| Vehicle | 38±1.91 | 177±3.31 |
| Toxicant (Scopolamine) 0.4 mg/kg | 58.16±1.34 | 146±6.831 |
| Standard (Piracetam) 200 mg/kg + Scopolamine | 38.66a,g±1.37 | 201.16a,g±6.44 |
| Test 10f 10 mg/kg + Scopolamine | 41.83a,e,g,k±1.34 | 187.6667a,g±12.93 |
| Test 10f 30mg/kg + Scopolamine | 41.83a,e,g,k±1.34 | 187a,g±14.88 |
Pole climbing chamber was used for passive avoidance response where pole was replaced by a wooden platform fixed on electric grid floor. Passive avoidance behavior based on negative reinforcement was used to examine the long-term memory. The apparatus was consisted of a box (27 cm×27 cm×27 cm) with three walls of plastic and front wall of plexiglass, featuring a grid floor (made up of 3 mm stainless steel rods set 8 mm apart), with a wooden platform (10 cm×7 cm×1.7 cm) in the center of the grid floor. Electric shock (6 mA) was delivered to the grid floor. The standard and test samples were administered orally to the respective groups for 8 consecutive days, whereas toxicant scopolamine hydrobromide was administered 90 min after the administration of the last dose on 8th day. The animals were exposed to the training session after 45 min of scopolamine hydrobromide administration. Training (i.e. 8th day of drug treatment) was carried out in two similar sessions. Each mouse was gently placed on the wooden platform set in the center of the grid floor. When the mouse was stepped-down placing all its paws on the grid floor, shocks were delivered for 15 sec and the step-down latency (SDL) was recorded. SDL was defined as the time (in seconds) taken by the mouse to step down from the wooden platform to grid floor with its paws on the grid floor. Animals which were showing SDL in the range of 2-15 seconds during the first test were used for the second session and the retention test. The second session was carried out 90 min after the first test. During second session, if the animals were stepped down before 60 sec, electric shocks were delivered once again for 15 sec. During the second test, animals were removed from shock free zone, if they did not step down for a period of 60 sec and were subjected to retention test. Retention memory was tested after 24 hr (i.e. 9th day, 24 hr after last dose) in a similar manner, except that the electric shocks were not applied to the grid floor observing an upper cut-off time of 300 sec and immediately animals in all the groups were humanely sacrificed using ether.
It was used to evaluate long term memory in mice based on negative reinforcement. In groups treated with test compound 10f (10 mg/kg and 30 mg/kg) significant increase in step down latency was observed as compared to toxicant group are shown in Table 4.
Acetylcholinesterase (AChE) In Vitro Assay for Cognition in Alzheimer’s Disease [29, 31]
A spectrophotometry method for determining acetyl cholinesterase activity of tissue extracts, homogenates and cell suspensions has been described. The principle of the method is the measurement of the rate of production of thiocholine from acetylthiocholine which is hydrolyzed in the presence of Acetyl cholinesterase (AChE). Further thiocholine react with 5, 5-dithiobis-2-nitrobenzoate ion (I) to produce the yellow anion of 5-thio-2-nitro-benzoic acid (II). The rate of formation of thiocholine from acetylthiocholine iodide in the presence of tissue cholinesterase was measured using spectrophotometer. It is based on coupling reactions:
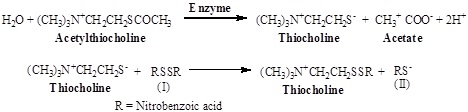
Reagents
- Substrate (Acetylthiocholine iodide 0.075 M): 21.67 mg Acetylthiocholine iodide was dissolved in 1 ml distilled water. This solution was used successfully for l0-15 days if it was refrigerated.
- Dithiobisnitrobenzoic acid (DTNB) 0.01M: 39.6 mg DTNB and 15 mg NaHCO3 were dissolved in 10 ml 0.1 M phosphate buffer (pH 7.4).
Procedure
- 0.4 ml of tissue homogenate was added to a cuvette containing 2.6 ml of phosphate buffer (pH 7.4, 0.1 M).
- Then DTNB reagent (100 μ1) was added to the cuvette. The absorbance was measured at 412 nm; when this had stopped increasing, the photometer slit was opened so that the absorbance was set to zero, then acetylthiocholine (20 μ1) was added. Changes in absorbance were recorded and the change in absorbance every minute for a period of 3 min was calculated.
- The rates of enzyme activity were calculated as follows:
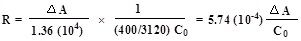
Where,
- R = Rate in moles substrate hydrolyzed per min per g of tissue
- ΔA = Change in absorbance per min
- C0 = Original concentration of tissue (mg/ml)
Statistical Analysis
The results of nootropic activity were expressed as mean ± SEM from 6 animals in each group. Results were statistically analyzed using one-way ANOVA following Turkey and Boferroni pos-hoc test; P<0.05 was considered significant. Graph Pad InStat version 4.00 of Graph Pad Software Inc. San Diego, USA was the software used for statistical analysis.
AChE enzyme inhibition would be potential target for prevention and treatment of cognition disorders. Test compound 10f treatment inhibited AChE activity in the brain significantly when compared to toxicant control. The effects of test compound on AChE inhibition were comparable to standard Piracetam treatment suggesting the anti-cholinesterase inhibitory activity of test compounds which might be the probable mechanism for its memory enhancing activity which are shown in Table 5.
Effect of Test compound on Acetyl cholinesterase level in Elevated plus maze and Passive avoidance paradigm models in mice.
| Groups | EPM(AchE in nmole/min/g tissue) n=3 | PAP (AchE in nmole/min/g tissue) n=3 |
|---|---|---|
| Vehicle | 207.98±4.58 | 212.5333±3.29 |
| Toxicant (Scopolamine) 0.4 mg/kg | 365.65±9.26 | 367.13±11.62 |
| Standard (Piracetam) 200 mg/kg + Scopolamine | 199.08a,g±9.77 | 196.98a,g±6.61 |
| Test 10f 10 mg/kg + Scopolamine | 223.34a,g±14.17 | 217.79a,g±4.50 |
| Test 10f 30mg/kg + Scopolamine | 223.52a,g±9.92 | 221.91a,g±14.44 |
Vehicle Vs Toxicant (EPM): F=2.179, p=0.2689 by One tailed ANOVA, p<0.0001 shown non significant result
Vehicle Vs Toxicant (PAP): F=12.466, p=0.0743 by One tailed ANOVA, p<0.0001 shown non significant result
Every group except vehicle control was treated with scopolamine
STRUCTURE-ACTIVITY RELATIONSHIP
To explore SAR, various substitutions were tried on R1 like alkyl, aryl, arylalkoxy, arylalkyl and aryl halo. All these substitutions were given potent binding affinities (Ki in nM).There was an increase in potency when introduced ethyl group instead of phenyl group at R1 (10f, Ki = 7.11 nM; 10a, Ki = 18.85 nM). N-alkylation on ring A was tolerated and maintained the binding affinity (10b, Ki = 20.34 nM; 10j, Ki = 24.73 nM). However, moving from on ring A without N-alkylation to methyl generally were reduced affinity (10d, Ki = 10.00 nM; 10l, Ki = 17.65 nM). In R1, ethyl, isopropyl and tolyl groups were among the most tolerated groups (10f, Ki = 7.11 nM; 10g, Ki = 8.71 nM and 10d, Ki = 10.00 nM). Obviously the substitution pattern on R1 compared to ring A was playing a role in the affinity potency of the compound. Variation of alkyl chain on R2 was not shown any significant change on binding affinity.
SUMMARY AND CONCLUSION
Novel, potent and safe 3-(1, 4-diazepanyl)-methyl-phenyl-sulphonamides as 5-HT6 receptor antagonists were designed using PHASE-ligand based drug designing approach and their binding affinities were evaluated, ADMET profiling by in silico screening. An efficient synthesis of these derivatives was carried out in good yields. Cognitive enhancing activity in Alzheimer’s disease and toxicity were evaluated by in vitro assay and in vivo animal models of screening.
It was confirmed earlier hypothesis about the importance of terminal nitrogen for binding to 5-HT6R. In groups treated with test compound 10f, transfer latency in EPM model was found to be significantly decreased as well as in Passive avoidance paradigm model, step down latency was found to be significantly increased as compared to groups treated with Scopolamine, which was indicated that the memory of learned task was retained in group treated with test compound 10f at 10 mg/kg and 30 mg/kg. Thus, our test compounds have an ability to prevent loss of memory. These results were further verified by evaluating effect of test compound on levels of acetylcholine in terms of cholinesterase activity where it was found to be decreased as compared to Scopolamine treated group. All these results were indicated that the observed memory retention effect in groups treated with our test compound probably were prevented metabolism of acetylcholine by inhibiting acetyl cholinesterase enzyme. Hence restoration of acetylcholine level in brain was led to retrivation of memory of learned task as observed in EPM and PAP test. Experimental results of synthesized derivatives were shown comparable activity to the earlier reported derivatives in literature on 5-HT6R therapeutic area. Future advanced pre-clinical development through SAR modifications to improve the overall pharmacokinetic parameters will be the future scope of the work.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors are indebted to Department of Biotechnology (DBT), New Delhi, India, for financial support and grateful to Prin. K. M. Kundanani College of Pharmacy, Cuffe Parade, Mumbai for providing splendid infrastructural facilities.














