RESEARCH ARTICLE
Extended Release of Timolol from Ethyl Cellulose Microparticles Laden Hydrogel Contact Lenses
Furqan A. Maulvi1, *, Tejal G. Soni2, Dinesh O. Shah3, 4, 5
Article Information
Identifiers and Pagination:
Year: 2015Volume: 2
First Page: 1
Last Page: 12
Publisher Id: PHARMSCI-2-1
DOI: 10.2174/1874844901502010001
Article History:
Received Date: 15/11/2014Revision Received Date: 18/2/2015
Acceptance Date: 9/3/2015
Electronic publication date: 14/4/2015
Collection year: 2015
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Glaucoma is a second leading cause of blindness globally after cataract, which is managed through eye drops, which are highly inefficient due to a low bioavailability of less than 1-5%. Frequent administration of eye drops leads to incompliance in patients, so there is a great need for medical device such as contact lenses to treat glaucoma. The objective of research was to provide sustained ocular delivery of timolol via prototype poly (hydroxyethyl methacrylate) hydrogel contact lenses which may improve bioavailability due to increase in ocular residence time of drug. The present work was to encapsulate drug in ethylcellulose microparticles, and to entrap these microparticles in the hydrogel. Microparticles were prepared by spray drying method using different ratios of drug to ethylcellulose. The solid state characterization studies of drug loaded microparticles revealed the transformation of drug to an amorphous state. The hydrogels were characterized by studying their optical and physical properties to determine their suitability as extended wear contact lenses. Microparticles laden hydrogels were compared with direct drug loaded hydrogels. The study of microparticles laden hydrogels showed reduction in optical and physical properties and the impact was proportional to the amount of microparticles in hydrogels. The results suggest the application of optimization and nanotechnology. In vitro drug release study revealed that direct loading batch delivers drug for 22 hours with high drug loading of 150 µg, while microparticles laden hydrogel deliver drug up to 48 hours (zero order kinetics) with low drug loading of 50 µg. The hydrogels appeared safe in the cytotoxicity study. The study demonstrated the promising potential of loading the ethyl cellulose microparticles into hydrogels to serve as a good platform for sustained ophthalmic drug delivery.
INTRODUCTION
More than 90% of ophthalmic drugs are delivered through eye drops. This route of administration is inefficient due to low bioavailability of less than 5%, and hence requires frequent instillation to maintain the drug concentration within the therapeutic window. Also, according to John C. Lang, the drug loss in the systemic circulation causes potential side effects [1-4].
Several systems have been developed in order to improve the pre-corneal residence time and penetration through the cornea, like in-situ gels and ointments which minimize nasolachrymal drainage but can cause blurred vision [5-8, 50]. Several polymeric drug delivery systems (DDS) have being developed, however, limitation of DDS includes poor chemical stability of liposomes, physical instability in nanoparticles, fusion in niosomes, complex synthetic route for dendrimers and limited drug loading capacity, etc. [9-11]. Implants can efficiently provide sustained drug delivery, but the risk of surgery limits their use [12]. According to a study performed by Himanshu Gupta in 2009 and other researchers, most of these systems sustained the drug release by only a few hours [13]. Therefore, there is a need for a new drug delivery system for sustained drug delivery.
The contact lens market globally comprises of daily disposables (58%), soft frequent replacement lens (8%), silicone hydrogels (28%), soft traditional lens (2%) and rigid lens (5%) [14, 15]. According to the annual ‘Trends in contact lens prescribing’ survey at the University of Manchester, soft lenses are dominated by daily disposables (45 %) and monthly replaced lenses (52 %), this survey clearly demonstrates an increase in usage of daily disposable soft contact lenses in today’s global market [16]. Contact lens can be used to prolong the ocular residence time of drug for more than 30 minutes. The increase in drug residence time leads to reduce drug wastage and increase bioavailability [17, 18]. Contact lens can be developed as an ideal medical device for ophthalmic drug delivery, as the drug residence time in the post-lens tear film (POLTF) is about 30 minutes, which is significantly higher than that of drugs delivered as eye drops, which is about 5 minutes [19-21].
Soft contact lenses were initially used for drug delivery by Sedlavek in 1965 [22, 23]. Soaking method was successfully used by Chi Chang, for ophthalmic drug delivery, but the major problem of loading drug by this method is that the loaded drug diffuses out in a very short time (few hours), which is inadequate for sustained drug delivery applications [1, 24].To improve/retard drug release durations, Chauhan et al. have proposed the development of nanoparticle laden gels that can load substantial amount of drug in the gel, which can be released at a controlled rate from the nanoparticles [25].Hiratani et al. focused on developing imprinted contact lenses [26, 27].Peng et al. have proposed the use of vitamin E for sustaining the drug delivery through contact lenses [28].Gulsen et al. encapsulated drugs in oil-in-water (o/w) microemulsions, and dispersed microemulsions in p-HEMA gels [29]. These systems delivered the drug for prolonged period of time, but suffered from several deficiencies including limitations on drug loading amount, short release durations, drug release during storage and impact on optical and physical properties of contact lens such as transparency, ion and oxygen permeability, etc. [30].
Therapeutic contact lenses for sustained drug delivery to treat glaucoma have being developed by many researchers. The lack of compliance associated with glaucoma therapy through eye drops could be potentially minimized by developing an extended release device, such as contact lenses. Thus, in addition to increase in bioavailability and reduced side effects, use of contact lens could improve patient adherence and thus led to better patient care and better clinical outcomes in glaucoma [2, 31-33]. This novel drug delivery method would also overcome cardiac side effects associated with timolol [34, 35].Timolol, a non-selective beta-adrenergic receptor antagonist, act by decreasing production of aqueous humor through blocking beta receptors in the ciliary body to reduce the intra ocular pressure [36].
The purpose of the study was to develop the timolol loaded ethyl cellulose microparticles laden hydrogel material that can be used as extended wear contact lens. To achieve this objective timolol loaded ethyl cellulose microparticles were prepared using spray drying technology and entrapped in hydrogel sheets. The microparticles loaded hydrogels were characterized to explore drug release kinetics, transparency, swelling, Na+ ion permeabilities and wettability. Furthermore, cytotoxicity studies are conducted to establish safety of particle loaded contact lenses. If such novel therapeutic hydrogel contact lenses are placed on the eye, the drug will slowly diffuse from the matrix of ethyl cellulose, travel through the lens matrix and enter the postlens and prelens tear film, with much longer residence time in the eye in comparison to eye drops. Thus the aim of the present study was to develop sustained release ophthalmic drug delivery system using timolol loaded ethyl cellulose microparticles laden hydrogel contact lenses.
MATERIALS AND METHODS
Materials
Hydroxyl ethyl methacrylate (HEMA), ethylene glycol dimethacrylate (EGDMA), methacrylic acid (MAA), ethyl cellulose 4cp, and Darocur® (2, 4, 6-trimethyl benzoyl-diphenyl-phosphinoxide) were purchased from Sigma-Aldrich Chemicals (MO, USA). Timolol maleate was received as a gift sample from Zydus Cadila Pharmaceuticals Ltd. (Gujarat, India). Immortalized rabbit corneal epithelial cell lines (Statens Seruminstitut Rabbit Cornea, ATCC® CCL60™) were purchased from National Centre for Cell Science (Pune, India). Ultra-pure water obtained by reverse osmosis (resistivity >18.2 MΩ cm; synergy U.V. Millipore, India) was used throughout the study. All the required chemicals were purchased from Sigma-Aldrich Chemicals (MO, USA).
Preparation of Timolol Loaded Ethyl Cellulose Microparticles
Timolol loaded ethyl cellulose microparticles were prepared using spray drying technique. Ethyl cellulose and timolol at various ratios (50:50, 70:30 and 85:15), were mixed and dissolved in ethanol. The clear solutions were spray dried using Mini Spray-dryer (Cronimac, India) to obtain drug dispersed ethyl cellulose microparticles. Applied spraying parameters were: 0.5-mm nozzle, inlet temperatures 80°C, outlet temperatures 50°C-60°C, spray-flow 5 kg/cm2, pumps setting 5 ml/min [37, 38]. Batches were coded as EC-50%, EC-70% and EC-85% for respective ratios of 50:50, 70:30 and 85:15 (ethyl cellulose: timolol).
Characterization of Ethyl Cellulose Microparticles
Dynamic light scattering: The size of Microparticles was determined using Malvern Zetasizer Nano ZS (Model ZEN 3500, WR, UK) at 633 nm. The Zetasizer uses the dynamic light scattering (DLS) principle to determine the particle size, and measurements were made using a detection angle of 90o [39].
Scanning electron microscope (SEM): To study the surface morphology of the microparticles, samples were analysed by SEM (JSM-6510LV, MA, USA). Silicon substrate (15 mm) was cleaned ultrasonically in acetone. SEM analysis was executed at 1 kV and magnifications ranging from 4000× to 12000× [40, 41].
Powder X-ray diffraction (PXRD): PXRD patterns were recorded at room temperature using Bruker’s model D8 Advance Diffractometer (Karlsruhe, West Germany) equipped with a compensating slit, using Cu-K radiation at 40 kV and 40 mA passing through nickel filter with divergence slit (0.5°), antiscattering slit (0.5°) and receiving slit (1 mm). Samples were scanned over a range of 2θ values from 10° to 80° at a scan rate of 0.02° sec-1 [42]. Average crystallite size of crystal was calculated based on PXRD peak broadening using the Scherrer equation (1).
Where τ is the mean crystallite dimension, K is a constant of 0.9, λ is the X-ray wavelength (1.542 nm), βτ is the peak broadening value due to crystal size reduction, i.e., the full-width-at-half-maximal (FWHM) [43].
Preparation Methods
Composition of pre-monomer mixture: Pre-monomer mixture includes hydroxyl ethyl methylacrylate (HEMA, 46.7% w/w) as common contact lens material, methacrylic acid (MAA, 0.8% w/w), ethylene glycol dimethylacrylate (EGDMA, 0.5% w/w) as cross-linking agents that add dimensional stability, and water (52% w/w).
Fabrication of prototype hydrogels: Hydrogels were fabricated by free radical polymerization reaction. 0.5% w/w of Darocur, a photo initiator was mixed with pre-monomer mixture. The mixtures were sonicated for 15 minutes to remove air bubbles and were transferred with help of micropipette (50 µL volume) between two glass plates (mould) separated by teflon spacer (0.1 mm thickness). The moulds were then placed in Ultraviolet transilluminator (Ultra Lum, Inc.) and the hydrogels were cured by irradiating UV-B light (365 nm) for 30 minutes. The hydrogels were then removed and preserved in desiccators till further use [44].
Direct loading of timolol into hydrogels: Timolol was loaded directly in hydrogels by dissolving timolol into pre-monomer mixture. Hydrogels were fabricated by the same procedure as described above. Three batches coded DL-50, DL-100, and DL-150 were prepared by adding 0.01%, 0.02%, and 0.03% w/w of timolol in pre-monomer mixture respectively. Each 50 μL volume of hydrogels was loaded with timolol, as shown in Table 1.
Details of timolol laden hydrogels by direct loading method.
| Coding | Timolol concentration in pre monomer mixture (%w/w) | Loading of timolol in each 50µL hydrogel (g)a |
|---|---|---|
| DL-50 | 0.01 | 52.54 ± 3.23 |
| DL-100 | 0.02 | 101.55 ± 4.67 |
| DL-150 | 0.03 | 153.75 ± 4.77 |
aThe data are based on the results of assay for individual hydrogels (not discussed in this paper). Values are mean ± standard deviation (n = 6).
Entrapment of timolol loaded ethyl cellulose microparticles into hydrogels: The microparticles laden hydrogels were fabricated by procedure as described above. To entrap timolol loaded microparticles in hydrogels, required amount of microparticles 0.2%, 0.33% and 0.66% w/w were added into pre monomer mixture for respective batches coded EC-50%, EC-70% and EC-85%, to achieve 50 µg of timolol loading in each 50 µL hydrogels.
Timolol was quantified using HPLC (Shimadzu, CA, USA). The separation column was Grace SmartTM C18 reverse-phase column (4.6 × 250 mm, 5 micron, California, USA), maintained at 40°C. The mobile phase was a mixture of phosphate buffer (pH 2.8) and methanol (65:35 v/v). The flow rate was fixed at 1 ml/min and the detection wavelength was set at 295 nm. The retention time for timolol under these conditions was 3.75 min. The standard curve of timolol in tear fluid was linear over the concentration range from 1-5 μg/ml (R2 = 0.999) [45, 46].
Transmittance: Optical property of hydrogel contact lenses should not change after loading timolol and microparticles in the hydrogels. The hydrogel sheets were hydrated by soaking in simulated tear fluid for 24 hours, and mounted in a quartz cuvette. The cuvette was placed in a UV–Vis spectrophotometer and at 630 nm transmittance was measured [47].
Swelling study: Swelling study is important in case of hydrogel contact lenses, as degree of hydration governs dimensions, oxygen permeability, transmittance and release rate of drug. Swelling study was conducted by placing hydrogels in simulated tear fluid at room temperature for 24 hrs. The hydrogels were removed from the solution, patted dry with a soft tissue paper, and weighed in air using a sensitive weighing balance. Initial dry weights of hydrogels were calculated directly after removing from the mold.
Na+ Ion permeability: Extended wear contact lenses must be permeable to ions to ensure homeostasis of ion concentration in the post lens tear film, which is necessary for lens motion. It has been reported that extended wear contact lenses must possess ion permeability greater than 1.5 × 10-6 mm2/min [39]. The ionic permeability of timolol and microparticles laden hydrogels was determined by experiment, in which hydrogel sheet was attached to the bottom of the donor chamber filled with 5 ml of 0.1M NaCl solution. Donor chamber consisted of a tube of 5 mm inner diameter, which was placed in a receiving chamber containing 50 ml of deionized water. The receiving chamber was placed on a magnetic stirring plate thermostatized at 35°C to simulate physiological conditions. The conductivity of receptor solution was monitored as a function of time using a conductivity meter and subsequently converted to NaCl concentration using the calibration plot. The conductivity probe was calibrated before each measurement experiment, using different NaCl solutions of known concentration ranging 1 μg/ml to 14 μg/ml. By plotting concentration of Na+ ions in receiving chamber versus time, the apparent Na+ ion permeability was calculated from the slope, using equation.
Where CR is the concentration of Na+ ions at time t in the receiving chamber, CL, 0 is the initial concentration of Na+ ions in donor chamber, A is the area of the lens exposed to the salt flux, L represents the average hydrogel thickness in the area exposed, VR is the volume of the receiving chamber compartment and P is the apparent permeability coefficient of Na+ ion [48].
Dynamic contact angle measurement: Contact angle was determined to evaluate the effect of timolol and microparticles on change in hydrophilicity of hydrogels (surface wettability) [49, 50]. Dynamic contact angle of Milli Q-water on timolol and microparticles laden dry hydrogels were determined using Theta Optical Goniometer (Attension, KSV Instrument, USA). Hydrogel sheet was placed on sample stage and with the help of Hamilton syringe a drop of Milli Q-water was placed on the surface of dry hydrogel. The captured image of drop was continuously analysed to measure the angle formed between the dry sheet and the tangent surface.
Drug release rate: Direct loaded and timolol loaded microparticles laden hydrogels were placed in 2 ml of simulated tear fluid in glass vial, and kept at 35°C in incubator with shaker at 100 RPM. The volume of the release medium was chosen to be 2 ml to approximately match the in vivo conditions of daily tear turnover. The simulated tear fluid was replaced at every interval with the same volume of fresh simulated tear fluid, to maintain perfect sink condition. The content of timolol was determined by HPLC at 295 nm after suitable dilution. The release profile of timolol was evaluated by plotting graphs of cumulative drug release (μg) versus time, percentage drug release versus time and release rate (ng/hr) versus time. The experiments were carried out in triplicate [51].
Mathematical model for diffusion coefficient and release kinetics: To determine the diffusion coefficient of timolol from hydrogels, the hydrogel sheet was assumed to be shaped like a slab [52]. The amounts released were fitted to equation (4) for short time (Mt/M∞ ˂ 0.65), of drug release profile.
By plotting the fractional release of timolol versus (t0.5/L), the diffusion coefficient was calculated from the slope. To evaluate how well the data matched with a Fickian release profile, the empirical Power Law equation (5) was used [53].
Using the data of timolol release profiles and by plotting graph of log (Mt/M∞) versus log (t), the diffusional exponent (n) was calculated from the slope and intercept k. By determining the diffusional exponent (n), the information about the physical mechanism controlling timolol release from a hydrogel was gain.
Estimation of therapeutic dose: For treatment of glaucoma the recommended dosage for 0.125% w/v solution of timolol eye drops is 1 drop 2 times daily. The volume of a typical eye drop is 50 μL. Thus a drop delivered two times a day would deliver 125 μg of timolol/day. The bioavailability of timolol delivered through eye drops is only about 5%, which implies that the therapeutic requirement of timolol from hydrogel contact lens is about 12.5 μg/day or 520 ng/hour (considering 50% bioavailability from contact lens) [39, 52].
Cytotoxicity study was conducted to check if the leachable extract from the microparticles laden hydrogels show any local toxicity. Immortalized rabbit corneal epithelial cell lines (Statens Seruminstitut Rabbit Cornea, ATCC® CCL60™) were purchased from National Centre for Cell Science (NCCS), Pune, India. Corneal epithelial cells were suspended in culture media (1×105 cells/ml) and seeded into 24-well plates (500 μl culture medium in each well). Culture medium includes Eagle minimum essential medium supplemented with 10% fetal calf serum, 100 μg/ml penicillin-streptomycin buffered with sodium bicarbonate, incubated at 5% CO2, 37°C, >90 % humidity for 24 h (∼ 1 doubling period) to form a semi-confluent monolayer. Culture medium was aspirated from the cells, and added per well, 500 μl of medium containing test extract (test extract was prepared in culture medium itself), or the negative control (without test extract), or the positive control (0.01 mg/ml Sodium Lauryl sulphate) and incubated for 24 hours (5% CO2, 37°C, > 90 % humidity). Commercially available methafilcon contact lenses (Bausch and Lomb, USA) composed of the same hydrogel material were purchased and used in study for comparison. After 24 hrs of incubation, cell viability was assessed using MTT dye (colorimetric assay). A volume of 50 μl of the MTT solution (0.5 mg/ml) was added to each test well and the plates were further incubated for 2 hrs in the incubator at 37°C. Formazan salt was solubilized in DMSO and absorbance was measured at 540 nm using MTT dye. Results are reported as mean ± standard deviation of measured absorbance normalized to the absorbance for non-treated control cells using equation (6). If viability is reduced to < 70 % of the negative control, it has a cytotoxic potential [54, 55].
Statistical analyses was carried out using t-test, analysis of variance (ANOVA) and the Tukey LSD test using SPSS programs (Version 21.0 for Window®, Chicago, IL).
RESULTS AND DISCUSSION
Characterization of Ethyl Cellulose Microparticles
Average particle size of batch EC-50%, EC-70% and EC-85% was found to be 4.33 µm, 4.82 µm and 4.59 µm respectively, which is in agreement with SEM report. The results clearly suggest that the particle size is uncorrelated to the amount of timolol loaded, and is instead controlled by the spray drying parameters (data not shown).
Surface morphology of timolol, ethyl cellulose and timolol loaded microparticles were studied by SEM (Fig. 1). In case of timolol, droplets of irregular shape were observed. Ethylcellulose was found to be porous and irregular in shape with nearly 200-1000 μm in size. SEM of microparticles for all three batches showed uniform spherical shaped porous (cavity) micron size particles (3-7 μm).
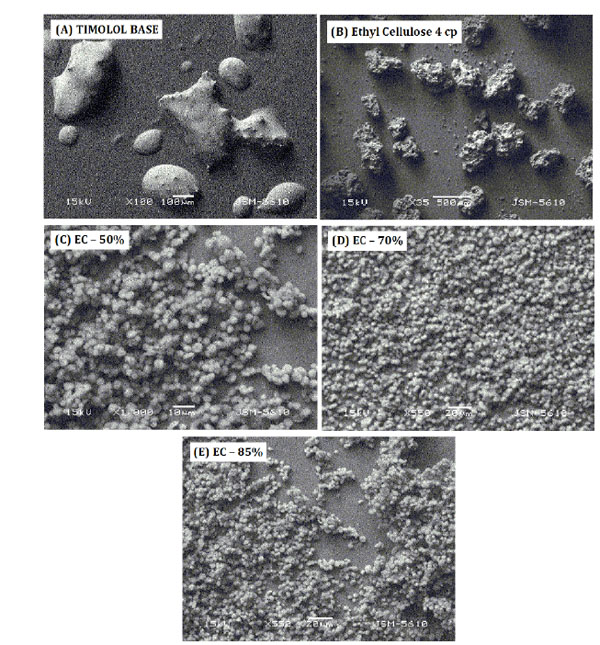 |
Figure 1. SEM report of (A) Timolol base, (B) Ethyl cellulose, (C) EC-50%, (D) EC-70%, (E) EC-85%. |
The XRD pattern of timolol, ethylcellulose and timolol loaded ethyl cellulose microparticles are shown in Fig. (2). The XRD scan of timolol showed number of intense peaks of crystallinity (highest peak at 2θ = 19.47, intensity = 4054); whereas the XRD pattern of microparticles exhibited a reduction in both number and intensity of peaks, indicating decrease in crystallinity and uniform dispersion of drug in ethylcellulose microparticles. The relative degree of crystallinity (RDC) was determined by comparing the representative peak height at 19.47 Pos. [R2θ] in the diffraction patterns of drug with those of drug loaded microparticles. The relationship used for the calculation of degree of crystallinity was (RDC) = Ip/Idrug, where Ip is the peak height of microparticles under investigation and Idrug is the peak height at the
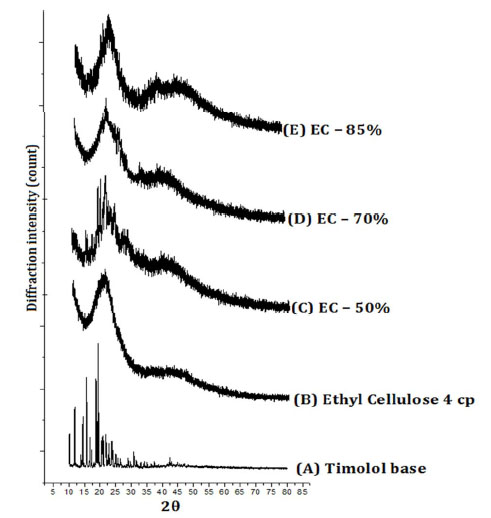 |
Figure 2. Powder XRD Pattern of (A) Timolol base, (B) Ethyl cellulose, (C) EC-50%, (D) EC-70%, (E) EC-85%. |
same angle for the drug with the highest intensity [40, 56-58]. The RDC for all samples are shown in Table 2. The order of reduction in the crystallinity is as follows: EC-50% > EC-70% > EC-85%. Also we can observe that, as the amount of ethyl cellulose increases, the crystallite size of drug reduces, indicating a better dispersion of drug in polymer. The change in d-spacing values indicates the doping of drug in planes of ethylcellulose (4.398Ao).
Results of powder XRD.
| Type of system | d-spacing [Å] at 20.17 [2] of Ethylcellulose (4 cP) | Intensity [cts] at 19.47[2Ø] |
RDC | Crystallite Size (nm) |
|---|---|---|---|---|
| Timolol base | - | 4054 | - | 671.77 |
| Ethylcellulose (4 cP) | 4.398 | - | - | - |
| EC - 50% | 4.134 | 1283 | 31.67 | 250.56 |
| EC - 70% | 4.244 | 875 | 21.58 | 81.61 |
| EC - 85% | 4.309 | 621 | 15.31 | 9.4 |
Transmittance
The % transmittance of hydrogels is important in its application as contact lenses for clear vision. Statistical results indicated that the direct drug laden hydrogels showed non-significant (P > 0.05) decrease in % transmittance (Table 3). Thus, transmittance was not affected in case of direct loading of timolol in hydrogels. The % transmittance was significantly reduced (p < 0.05), below 90% for microparticles laden batches. The impact was directly proportional to amount of microparticles loaded in hydrogels. There was no change in % transmittance, if amount of timolol in microparticles was varied (data not shown). The reduction in transmittance of hydrogel is due to larger particle size (4 to 5 microns); this problem can be resolved by reducing the particle size to nano level to improve visual properties. Thus the presence of hydrophobic ethylcellulose microparticles reduced the visual clarity of hydrogels, which is not good enough for contact lens use.
Swelling
Swelling study is critical in case of hydrogel contact lenses, as degree of hydration governs dimensions, ion permeability, oxygen permeability, and release of drug from contact lens. The data of % swelling are shown in Table 3. The % swelling was slightly decreased with increase in timolol in direct timolol loaded batches. Statistical analysis results showed no significant difference (P > 0.05) in % swelling between the blank hydrogel and direct timolol loaded hydrogels. In case of timolol loaded ethylcellulose microparticles laden hydrogels, the % swelling was expected to decrease due to negligible water uptake by hydrophobic microparticles. Statistical analysis results showed significant decrease (P < 0.05) in % swelling between the blank hydrogels and microparticles laden hydrogels. Also % swelling decreases as the amount of microparticles increases (from Batch EC-50% to EC-85%). Thus the optimization of the amount of microparticles in hydrogel is prerequisite to study, so that the % swelling do not affects the dimension and other vital properties of contact lens.
Physical properties of hydrogel sheets.
| Hydrogel type | Transmittance (%) | Swelling (%) | Na+ ion permeability [Pion 10-6(mm2/min)] |
|---|---|---|---|
| Blank sheet | 98.3 ± 0.05 | 78.50 ± 0.99 | 6.38 ± 0.19 |
| DL-50 | 98.1 ± 0.10 | 77.61 ± 1.10 | 5.75 ± 0.25 |
| DL-100 | 97.6 ± 0.08 | 76.49 ± 1.39 | 5.14 ± 0.37 |
| DL-150 | 97.3 ± 0.09 | 75.22 ± 1.47 | 4.67 ± 0.26 |
| EC-50% | 86.9 ± 0.25 | 76.30 ± 0.84 | 4.16 ± 0.61 |
| EC-70% | 78.8 ± 0.55 | 70.72 ± 0.78 | 3.76 ± 0.35 |
| EC-85% | 62.4 ± 0.70 | 62.82 ± 0.84 | 2.06 ± 0.57 |
Na+ Ion Permeability
Adequate Na+ ion permeability is required to prevent abrasion of contact lens to the eye by forming a fluid hydrodynamic boundary layer between the contact lens and the cornea. According to Nicolson, the minimum requirement for Na+ ion permeability (Pion) should be 1.5 x 10-6 mm2/min. The experimental values of apparent Na+ ion permeability were obtained from equation (3)following the experimental procedure described in method.The values of apparent Na+ ion permeability for all the hydrogels are listed in Table 3. The values of Na+ ion permeability for direct timolol loaded batches decreased significantly (p < 0.05) in comparison to blank hydrogels, which could be due to the presence of hydrophobic timolol base.
Furthermore Na+ ion permeability for microparticles laden batches decreased significantly (P > 0.05) with increase in microparticles loading (from batch EC-50% to EC-85%), in comparison to blank hydrogels i.e., the presences of hydrophobic drug and ethyl cellulose microparticles in hydrogels interfere with Na+ ion permeability. Though the values are above minimum requirement, i.e. 1.5 x 10-6 mm2/min, to assure that the contact lenses prepared from such hydrogel materials would not cause abrasion to cornea upon insertion.
Wettability of contact lens is an important property related to the wearing comfort for user. Wetting behavior was predicted by measuring the dynamic surface contact angle formed by a drop of Milli Q water on the surface of dry hydrogel sheet [50]. The change in hydrophilicity of the surface of dried hydrogels after incorporating timolol and microparticles were evaluated through contact angle measurements. Dynamic contact angles (θ) of Milli Q-water on timolol and microparticles laden hydrogel sheets are presented in Fig. (3). In direct timolol loading batches, there is small increase in contact angle at initial 10 seconds with increase in timolol loading. Statistical results indicated insignificant (p > 0.05) increased in contact angle in comparison to bank hydrogels. Thus the presence of hydrophobic timolol base did
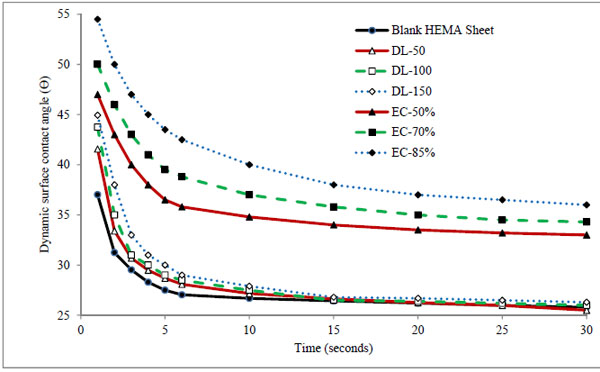 |
Figure 3. Dynamic surface contact angle of Milli Q-water on blank and formulation laden dry hydrogels. |
not affect the wetting behavior of hydrogels significantly, which was also supported by the results of swelling, and transmittance. For microparticles laden dry hydrogels, a significant increased (p < 0.01) in contact angle was observed in comparison to blank and direct timolol loaded hydrogels. Contact angle was also increased with amount of microparticles in hydrogels (from 0.2% to 0.66%w/w for respective batches EC-50% to EC-85%). The results confirmed that the hydrophobic polymer ethylcellulose do play a significant role in decreasing the wettability of hydrogels likewise swelling, transmittance and ion permeability behavior. Therefore the optimal level of microparticles in hydrogel is required, so that it does not affect the vital properties of contact lens for comfort use. Although there is an increase in contact angle in the presence of the microparticles, the value is still below 900, so the surface is still hydrophilic in nature. The results suggest that, we need to hydrate the hydrogels for longer duration of time for complete hydration before use.
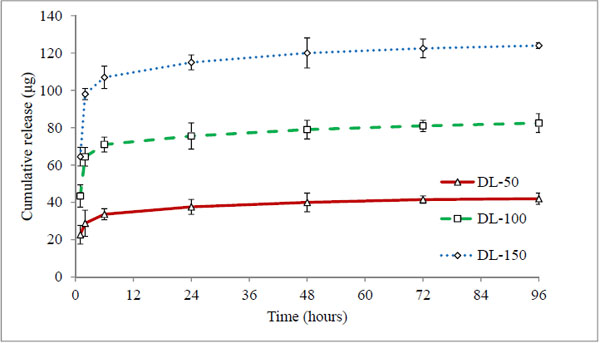
Conventional methodology of direct drug loading was achieved by adding timolol base directly into pre-monomer mixture (direct loading), before fabrication. Three hydrogels coded DL-50, DL-100 and DL-150 were analysed, which contain 50 μg, 100 μg, and 150 μg of drug respectively (in each 50 μL volume of hydrogel). The cumulative (μg) and percentage drug release profiles are shown in Figs. (4 and 5) respectively.
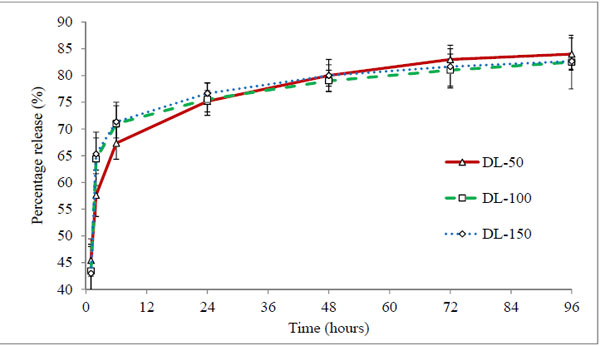 |
Figure 5. Percentage release profile of timolol loaded hydrogels prepared by direct loading method. Values are shown as mean ± standard deviation (n=6). |
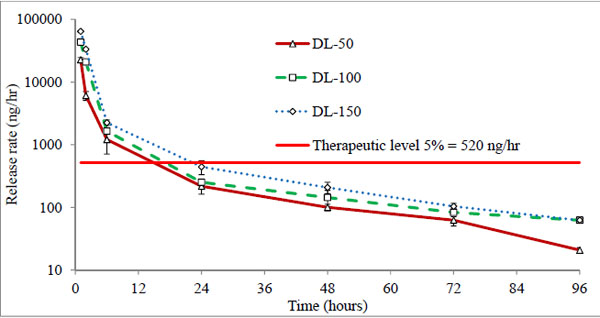
All three batches showed a burst release (43-45%) at first hour (Fig. 5), indicating rapid diffusion of drug from the aqueous channels of hydrogel matrix. This could be due to partial solubility of timolol in tear fluid (2.74 mg/ml) and absence of controlling membrane. The cumulative drug release profiles suggest that the amount of drug released depends on the drug loading in hydrogels, while percentage drug release from hydrogels with time is independent of the amount of drug loaded (non-significant, p > 0.05). All the hydrogels under study displayed the same general pattern of drug release profiles. Release rate of timolol within the therapeutic level (520 ng/hour) for DL-50, DL-100 and DL-150 batches were found to be 14, 15 and 22 hours respectively (Table 4, Fig. 6). Thus with direct loading methodology release up to 22 hours is possible, with limitation of high burst release and high drug loading.
Drug release kinetics data of direct loaded and timolol loaded ethyl cellulose microparticles laden hydrogels.
| Hydrogels | Average drug Loading (µg) | Release within therapeutic level (520 ng/hr), (Hours) |
Diffusion coefficient (D = 10-7 mm2/sec) |
Regression coefficient (R2) |
Diffusional exponent (n) |
Intercept k |
|---|---|---|---|---|---|---|
| DL-50 | 50 | 14 | 2.36 | 0.93 | 0.212 | 1.67 |
| DL-100 | 100 | 15 | 3.31 | 0.79 | 0.257 | 1.67 |
| DL-150 | 150 | 22 | 3.44 | 0.77 | 0.263 | 1.67 |
| EC-50% | 50 | 20 | 2.68 | 0.97 | 0.468 | 1.46 |
| EC-70% | 50 | 30 | 1.89 | 0.99 | 0.468 | 1.17 |
| EC-85% | 50 | 48 | 1.57 | 0.99 | 0.651 | 0.76 |
To understand the mechanism of drug release, it was imperative to compare the short span release profiles (65% release) with diffusion controlled models. Release profiles were fitted to equation 4 and 5 to give diffusion coefficient values, release exponent n and intercept k as shown in Table 4. The calculated diffusion coefficients (Average thickness of sheet = 0.47 mm) for batches DL-50, DL-100 and DL-150 were 2.36 × 10-7, 3.31 × 10-7, and 3.44 × 10-7mm2/sec and the diffusional exponent (n) was found to be 0.212, 0.257 and 0.263 respectively. Diffusion coefficient values actually increases with increase in timolol loading, suggesting no added advantage of increased in drug loading for sustained drug delivery. The diffusional exponents (n < 0.5) signpost diffusion controlled fickian release of timolol from hydrogels for all three batches. Higher values of intercept k, indicate low affinity of timolol with hydrogel polymer matrix, and no change (p>0.05) in k values indicate that there is no change in affinity between timolol and hydrogel polymer matrix with increased in drug loading.
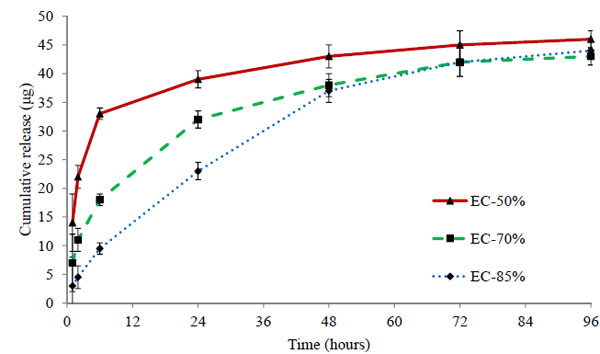
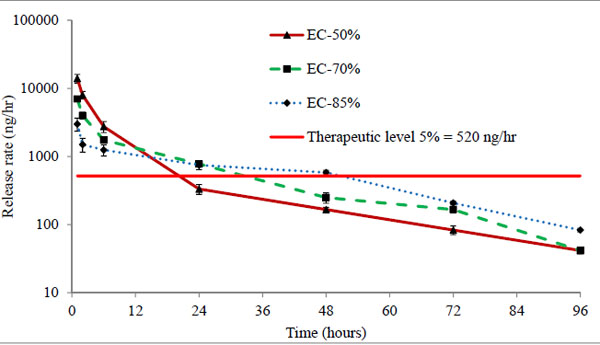
Three batches EC-50%, EC-70%, and EC-85% containing 50 μg of timolol in each 50 μL volume of hydrogels were analysed. The cumulative (μg) and percentage release profiles are shown in Figs. (7 and 8) respectively. Ethyl cellulose content in microparticles increases from 50% to 85% for respective batches EC-50% to EC-85%. Release profile curves indicated that as the amount of ethyl cellulose in microparticles increases, initial burst release reduces showing more sustained release of drug. Thus, the percentage release of timolol from hydrogel with time is dependent (P < 0.05) on the amount of ethyl cellulose in microparticles. A significant enhancement (p < 0.01) in release rate duration was observed (Fig. 9), from batch EC-50% to EC-85%. Release rate of timolol within the therapeutic level (520 ng/hr) for EC-50%, EC-70%, and EC-85% batches were found to be 20 hrs, 30 hrs and 48 hrs respectively (Table 4).
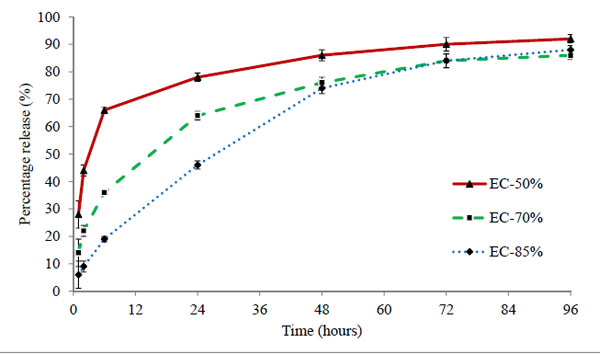 |
Figure 8. Percentage release profile of timolol from ethyl cellulose laden hydrogels prepared by spray drying method. Values are shown as mean ± standard deviation (n=6). |
The mechanism of drug transport from microparticles laden hydrogel to release media is highly complex, potentially involving drug diffusion, drug dissolution, drug-polymer chain interactions, time-dependent drug permeability within the polymeric matrix, etc [39]. The possible barrier involved in restricting the drug release could be the long tortuous pathway created by the matrix of ethylcellulose. Also higher the amount of ethylcellulose in microparticles, longer the pathway and more sustained release observed (EC-85% batch). The microparticles laden hydrogels showed sustained release even with low drug loading of 50µg in comparison to direct loading batches (50-150 µg). Thus the results clearly suggest the potential of ethyl cellulose microparticles in sustaining the drug release duration.
Diffusion coefficient for EC-50%, EC-70%, and EC-85% batches were found to be 2.68 × 10-7, 1.89 × 10-7 and 1.57 × 10-7 mm2/sec and the diffusional exponents (n) 0.468, 0.468, and 0.651 respectively (Table 4). Diffusion coefficient differ significantly (p<0.01) in comparison to direct drug loaded hydrogels. Diffusion of drug from hydrogel decreased with increase in amount of ethyl cellulose in microparticles. The diffusional exponents for EC-85% batch, signpost that we successfully achieved zero order release kinetics. Lower values of intercept k in comparison to direct drug loaded hydrogels (p < 0.05); clearly suggest a very strong affinity of timolol with ethyl cellulose in hydrogel matrix. Also the affinity significantly increases (p <0.01) with increase in ethyl cellulose in microparticles. The results suggest that increase in ethyl cellulose content in microparticles, increases the drug release duration from hydrogel, while at the same time affecting the physical and optical property of hydrogels. The results propose significant improvement in release duration (sustained release) with timolol loaded ethyl cellulose microparticles laden hydrogels prepared by spray drying method in comparison with direct loading method.
Cytotoxicity could result from extracts of timolol loaded ethyl cellulose microparticles, or other factors. Cytotoxicity was assessed by incubating cells in media that contained an extracts of microparticles, blank hydrogel, commercial methafilcon contact lens, vehicle (media only, control group). After 24 hrs of exposure, cellular viability was assessed using an MTT colorimetric assay. Compared to cells that were exposed to media only, batch EC-50%, EC-70%, EC-85% and methafilcon contact lens demonstrated cell viabilities of 93.45 ± 5.42%, 96.45 ± 6.42%, 94.45 ± 4.42% and 94.65 ± 2.18% respectively (Table 5). The viability of cells exposed to extracts of microparticles was not statistically different (p> 0.05) from methafilcon and blank hydrogels. The percentage viability was greater than 70%, thus the microparticles laden hydrogel material was found to be safe for contact lens wear, and can be used for further animal study.
Cell viability of statens seruminstitut rabbit corneal cells
| Hydrogel Type | % Cell Viability (mean ± SD, n=4) |
|---|---|
| SLS (0.01mg/ml), Positive control | 56.51 ± 0.42 |
| Methafilcon lens | 94.65 ± 2.18 |
| Blank hydrogel | 95.60 ± 3.42 |
| EC-50% | 93.45 ± 5.42 |
| EC-70% | 96.45 ± 6.42 |
| EC-85% | 94.45 ± 4.42 |
CONCLUSION
The study demonstrates the limitation of direct loading method to sustain the ophthalmic drug release duration through hydrogel contact lenses. While microparticles laden hydrogels (EC-85%) showed zero order release kinetics, likely due to ethylcellulose (hydrophobic polymer) which forces the drug molecules to diffuse through long tortuous pathway from microparticles. The hydrogels appeared safe in cytotoxicity study. However, the optical and physical properties of contact lenses were affected due to large particle size in micron range, suggesting the need of nanotechnology and optimization. > From this research work, it can be concluded that the concept of loading drug loaded ethyl cellulose microparticles into hydrogels has a strong potential to serve as a good platform for sustained ophthalmic drug delivery.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This research project was funded by LeoLens Technology, San Diego, California 92128, USA. The authors are grateful to Dr. Akshay R. Koli and Dr. Ketan M. Ranch, Assistant Professors, Maliba Pharmacy College, Gujarat, India for their technical assistance and to Dr. Bhavin A. Vyas, Associate Professor, HOD, Pharmacology Department, Maliba Pharmacy College, for his guidance and cooperation in cytotoxicity study. The author is also thankful to Dr. Renu S. Chauhan, Registrar, Uka Tarsadia University, for her suggestions in language editing.